Differential physiological roles for BIN1 isoforms in skeletal muscle development, function and regeneration
- PMID: 32994313
- PMCID: PMC7710016
- DOI: 10.1242/dmm.044354
Differential physiological roles for BIN1 isoforms in skeletal muscle development, function and regeneration
Abstract
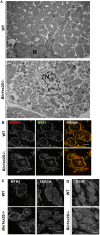
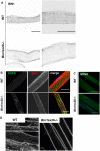
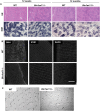
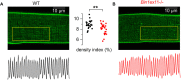
Skeletal muscle development and regeneration are tightly regulated processes. How the intracellular organization of muscle fibers is achieved during these steps is unclear. Here, we focus on the cellular and physiological roles of amphiphysin 2 (BIN1), a membrane remodeling protein mutated in both congenital and adult centronuclear myopathies (CNM), that is ubiquitously expressed and has skeletal muscle-specific isoforms. We created and characterized constitutive muscle-specific and inducible Bin1 homozygous and heterozygous knockout mice targeting either ubiquitous or muscle-specific isoforms. Constitutive Bin1-deficient mice died at birth from lack of feeding due to a skeletal muscle defect. T-tubules and other organelles were misplaced and altered, supporting a general early role for BIN1 in intracellular organization, in addition to membrane remodeling. Although restricted deletion of Bin1 in unchallenged adult muscles had no impact, the forced switch from the muscle-specific isoforms to the ubiquitous isoforms through deletion of the in-frame muscle-specific exon delayed muscle regeneration. Thus, ubiquitous BIN1 function is necessary for muscle development and function, whereas its muscle-specific isoforms fine tune muscle regeneration in adulthood, supporting that BIN1 CNM with congenital onset are due to developmental defects, whereas later onset may be due to regeneration defects.
Keywords: Animal model; BAR domain; Centronuclear myopathy; Dynamin; Myoblast fusion; Myotonic dystrophy; Myotubular myopathy; SH3 domain; Triad; XLMTM.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsB.S.C. and J. Laporte are co-founders of Dynacure, and B.S.C. is a Dynacure employee.
Figures



References
-
- Al-Qusairi L., Weiss N., Toussaint A., Berbey C., Messaddeq N., Kretz C., Sanoudou D., Beggs A. H., Allard B., Mandel J.-L. et al. (2009). T-tubule disorganization and defective excitation-contraction coupling in muscle fibers lacking myotubularin lipid phosphatase. Proc. Natl. Acad. Sci. USA 106, 18763-18768. 10.1073/pnas.0900705106 - DOI - PMC - PubMed
-
- Amoasii L., Bertazzi D. L., Tronchère H., Hnia K., Chicanne G., Rinaldi B., Cowling B. S., Ferry A., Klaholz B., Payrastre B. et al. (2012). Phosphatase-dead myotubularin ameliorates X-linked centronuclear myopathy phenotypes in mice. PLoS Genet. 8, e1002965 10.1371/journal.pgen.1002965 - DOI - PMC - PubMed
-
- Blondelle J., Ohno Y., Gache V., Guyot S., Storck S., Blanchard-Gutton N., Barthélémy I., Walmsley G., Rahier A., Gadin S. et al. (2015). HACD1, a regulator of membrane composition and fluidity, promotes myoblast fusion and skeletal muscle growth. J. Mol. Cell Biol. 7, 429-440. 10.1093/jmcb/mjv049 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

