Sex Steroids Induce Membrane Stress Responses and Virulence Properties in Pseudomonas aeruginosa
- PMID: 32994320
- PMCID: PMC7527723
- DOI: 10.1128/mBio.01774-20
Sex Steroids Induce Membrane Stress Responses and Virulence Properties in Pseudomonas aeruginosa
Erratum in
-
Erratum for Vidaillac et al. "Sex Steroids Induce Membrane Stress Responses and Virulence Properties in Pseudomonas aeruginosa".mBio. 2020 Nov 3;11(6):e02809-20. doi: 10.1128/mBio.02809-20. mBio. 2020. PMID: 33144381 Free PMC article. No abstract available.
Abstract
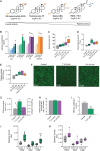
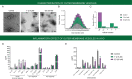
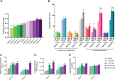
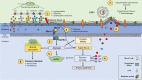
Estrogen, a major female sex steroid hormone, has been shown to promote the selection of mucoid Pseudomonas aeruginosa in the airways of patients with chronic respiratory diseases, including cystic fibrosis. This results in long-term persistence, poorer clinical outcomes, and limited therapeutic options. In this study, we demonstrate that at physiological concentrations, sex steroids, including testosterone and estriol, induce membrane stress responses in P. aeruginosa This is characterized by increased virulence and consequent inflammation and release of proinflammatory outer membrane vesicles promoting in vivo persistence of the bacteria. The steroid-induced P. aeruginosa response correlates with the molecular polarity of the hormones and membrane fluidic properties of the bacteria. This novel mechanism of interaction between sex steroids and P. aeruginosa explicates the reported increased disease severity observed in females with cystic fibrosis and provides evidence for the therapeutic potential of the modulation of sex steroids to achieve better clinical outcomes in patients with hormone-responsive strains.IMPORTANCE Molecular mechanisms by which sex steroids interact with P. aeruginosa to modulate its virulence have yet to be reported. Our work provides the first characterization of a steroid-induced membrane stress mechanism promoting P. aeruginosa virulence, which includes the release of proinflammatory outer membrane vesicles, resulting in inflammation, host tissue damage, and reduced bacterial clearance. We further demonstrate that at nanomolar (physiological) concentrations, male and female sex steroids promote virulence in clinical strains of P. aeruginosa based on their dynamic membrane fluidic properties. This work provides, for the first-time, mechanistic insight to better understand and predict the P. aeruginosa related response to sex steroids and explain the interindividual patient variability observed in respiratory diseases such as cystic fibrosis that are complicated by gender differences and chronic P. aeruginosa infection.
Keywords: Pseudomonas aeruginosa; gender; hormones; membrane stress; steroids.
Copyright © 2020 Vidaillac et al.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

