Antibodies from Sierra Leonean and Nigerian Lassa fever survivors cross-react with recombinant proteins representing Lassa viruses of divergent lineages
- PMID: 32994446
- PMCID: PMC7525497
- DOI: 10.1038/s41598-020-72539-w
Antibodies from Sierra Leonean and Nigerian Lassa fever survivors cross-react with recombinant proteins representing Lassa viruses of divergent lineages
Abstract
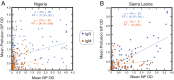
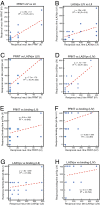
Lassa virus (LASV) is the causative agent of Lassa fever, an often-fatal hemorrhagic disease that is endemic in West Africa. Seven genetically distinct LASV lineages have been identified. As part of CEPI's (Coalition for Epidemic Preparedness Innovations) Lassa vaccine development program, we assessed the potential of the human immune system to mount cross-reactive and cross-protective humoral immune responses to antigens from the most prevalent LASV lineages, which are lineages II and III in Nigeria and lineage IV in Sierra Leone. IgG and IgM present in the blood of Lassa fever survivors from Nigeria or Sierra Leone exhibited substantial cross-reactivity for binding to LASV nucleoprotein and two engineered (linked and prefusion) versions of the glycoproteins (GP) of lineages II-IV. There was less cross-reactivity for the Zinc protein. Serum or plasma from Nigerian Lassa fever survivors neutralized LASV pseudoviruses expressing lineage II GP better than they neutralized lineage III or IV GP expressing pseudoviruses. Sierra Leonean survivors did not exhibit a lineage bias. Neutralization titres determined using LASV pseudovirus assays showed significant correlation with titres determined by plaque reduction with infectious LASV. These studies provide guidance for comparison of humoral immunity to LASV of distinct lineages following natural infection or immunization.
Conflict of interest statement
Tulane University and its various academic and industry partners have filed US and foreign patent applications for countermeasures to emerging viruses. Technical information may also be kept as trade secrets. If commercial products are developed, including Lassa diagnostics, MLH, MLB, DSKN, DJB, RWC, APK, KMH, MMR, IA, SK, RL, JGS, JSS, TWG, EOS, CTH, DSG, RFG and LMB may receive royalties or profits. This does not alter our adherence to all policies of the NIH and
Figures





References
-
- McCormick JB. Epidemiology and control of Lassa fever. Curr. Top Microbiol. Immunol. 1987;134:69–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

