Camera-based optical palpation
- PMID: 32994500
- PMCID: PMC7524728
- DOI: 10.1038/s41598-020-72603-5
Camera-based optical palpation
Abstract
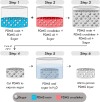
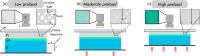
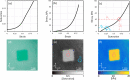
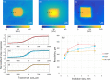
Optical elastography is undergoing extensive development as an imaging tool to map mechanical contrast in tissue. Here, we present a new platform for optical elastography by generating sub-millimetre-scale mechanical contrast from a simple digital camera. This cost-effective, compact and easy-to-implement approach opens the possibility to greatly expand applications of optical elastography both within and beyond the field of medical imaging. Camera-based optical palpation (CBOP) utilises a digital camera to acquire photographs that quantify the light intensity transmitted through a silicone layer comprising a dense distribution of micro-pores (diameter, 30-100 µm). As the transmission of light through the micro-pores increases with compression, we deduce strain in the layer directly from intensity in the digital photograph. By pre-characterising the relationship between stress and strain of the layer, the measured strain map can be converted to an optical palpogram, a map of stress that visualises mechanical contrast in the sample. We demonstrate a spatial resolution as high as 290 µm in CBOP, comparable to that achieved using an optical coherence tomography-based implementation of optical palpation. In this paper, we describe the fabrication of the micro-porous layer and present experimental results from structured phantoms containing stiff inclusions as small as 0.5 × 0.5 × 1 mm. In each case, we demonstrate high contrast between the inclusion and the base material and validate both the contrast and spatial resolution achieved using finite element modelling. By performing CBOP on freshly excised human breast tissue, we demonstrate the capability to delineate tumour from surrounding benign tissue.
Conflict of interest statement
B.F.K., C.M.S., A.C. and B.L. hold shares in OncoRes Medical, a startup company developing optical coherence elastography for surgical applications. B.F.K. and A.C. receive research funding from this company. The other authors declare no conflicts of interest related to this article.
Figures

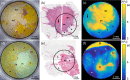
References
-
- Kennedy BF, Wijesinghe P, Sampson DD. The emergence of optical elastography in biomedicine. Nat. Photonics. 2017;11:215. doi: 10.1038/nphoton.2017.6. - DOI
-
- Kennedy BF, Kennedy KM, Sampson DD. A review of optical coherence elastography: Fundamentals, techniques and prospects. IEEE J. Sel. Top. Quant. 2014;20:272–288. doi: 10.1109/JSTQE.2013.2291445. - DOI
-
- Zaitsev, V. Y. et al. Strain and elasticity imaging in compression optical coherence elastography: the two-decade perspective and recent advances. J. Biophotonicsn/a, e202000257. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

