Real-life effectiveness of mepolizumab in patients with severe refractory eosinophilic asthma and multiple comorbidities
- PMID: 32994855
- PMCID: PMC7508691
- DOI: 10.1016/j.waojou.2020.100462
Real-life effectiveness of mepolizumab in patients with severe refractory eosinophilic asthma and multiple comorbidities
Abstract
Background: Data on mepolizumab in patients with severe eosinophilic asthma (EA) and comorbidities are needed to assess whether randomized controlled trial results are applicable in the real world.
Objective: To evaluate real-life effectiveness and the presence/absence of predictors of treatment response in patients with one or more comorbidities (nasal polyps, allergic rhinitis, gastro-esophageal reflux disease, nonallergic rhinitis with eosinophilia syndrome, obesity, bronchiectasis) who received mepolizumab (MEPO) for the treatment of severe EA.
Methods: We performed a single-center retrospective study in patients with severe asthma and presence of comorbidities treated with mepolizumab at the respiratory outpatient clinic, Policlinico-Vittorio Emanuele, Catania, Italy. Health records of 31 severe asthmatic patients were retrieved and analyzed. Asthma control test (ACT) score, blood eosinophil count, forced expiratory volume in 1 s (FEV1), FEV1% of predicted and FEV1/FVC (Forced Vital Capacity) ratio, oral corticosteroid (OCS) dosage, and exacerbations were recorded at baseline (T0), after 3 (T1), 6 (T3), 9 (T6), and 12 months (T12). Clinical response was defined when 3 of these 4 criteria were fulfilled: i) 30% exacerbation decrease; ii) 80% blood eosinophilia reduction; iii) 3 point ACT increase; iv) FEV1 increase ≥200 mL.
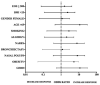
Results: 83.87% of patients were classified as responsive to MEPO treatment. Substantial depletion of the blood eosinophils (>80%) was found in 87.1% of patients, FEV1 > 200 mL was seen in 54.84% of patients, a 3-point ACT improvement from baseline was recorded in 80.65% 25 of patients and a 30% reduction of exacerbations rates was seen in 96.77% of patients. Moreover, the majority 38.71% of patients met 3/4 parameters after 12 months. Neither the comorbidities nor other characteristics (sex, BMI, age, smoking) influenced treatment response.
Conclusions: MEPO in patients with severe EA is effective regardless of the presence of comorbidities.
Keywords: ACT, Asthma Control Test; BMI, Body Mass Index; DREAM, Dose Ranging Efficacy And safety with Mepolizumab; EA, Eosinophilic Asthma; ECRS, Eosinophilic Chronic Rhinosinusitis; ERS/ATS, European Respiratory Society/American Thoracic Society; FEV1, Forced Expiratory Volume in 1 s; FEV1/FVC, Forced Expiratory Volume in 1 s/Forced Vital Capacity ratio; FVC, Forced Vital Capacity; GERD, Gastro-Esophageal Reflux Disease; GINA, Global INitiative for Asthma; IL-5, Interleukin-5; IQR, Interquartile Range; IgG, Immunoglobulin G; MEPO, Mepolizumab; Mepolizumab; Multiple comorbidities; NARES, Non Allergic Rhinitis with Eosinophilia Syndrome; OCS, Oral Corticosteroid; RCTs, Randomized Controlled Trials; RV, Residual Volume; SD, Standard Deviation; SEM, Standard Error Mean; Severe eosinophilic asthma; T0, baseline; T1, 3 months after baseline; T12, 12 months after baseline; T3, 6 months after baseline; T6, 9 months after baseline.
© 2020 The Author(s).
Conflict of interest statement
All the authors declare no competing interests.
Figures

References
-
- Chung K.F., Wenzel S.E., Brozek J.L. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. - PubMed
-
- European Medicine Agency (Ema). https://www.ema.europa.eu/en/documents/overview/nucala-epar-medicine-ove... accessed 25 november 2019.
-
- Chupp G.L., Bradford E.S., Albers F.C. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390–400. - PubMed
-
- Pavord I.D., Korn S., Howarth P. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. - PubMed
-
- Ortega H.G., Liu M.C., Pavord I.D. MENSA Investigators. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

