Enhancing the efficacy of immunotherapy using radiotherapy
- PMID: 32994997
- PMCID: PMC7507442
- DOI: 10.1002/cti2.1169
Enhancing the efficacy of immunotherapy using radiotherapy
Abstract
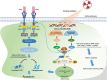
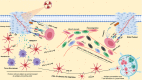
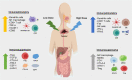
Recent clinical breakthroughs in cancer immunotherapy, especially with immune checkpoint blockade, offer great hope for cancer sufferers - and have greatly changed the landscape of cancer treatment. However, whilst many patients achieve clinical responses, others experience minimal benefit or do not respond to immune checkpoint blockade at all. Researchers are therefore exploring multimodal approaches by combining immune checkpoint blockade with conventional cancer therapies to enhance the efficacy of treatment. A growing body of evidence from both preclinical studies and clinical observations indicates that radiotherapy could be a powerful driver to augment the efficacy of immune checkpoint blockade, because of its ability to activate the antitumor immune response and potentially overcome resistance. In this review, we describe how radiotherapy induces DNA damage and apoptosis, generates immunogenic cell death and alters the characteristics of key immune cells in the tumor microenvironment. We also discuss recent preclinical work and clinical trials combining radiotherapy and immune checkpoint blockade in thoracic and other cancers. Finally, we discuss the scheduling of immune checkpoint blockade and radiotherapy, biomarkers predicting responses to combination therapy, and how these novel data may be translated into the clinic.
Keywords: apoptosis; immune checkpoint blockade; immunogenic cell death; radiotherapy.
© 2020 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The Emergence of Immune-checkpoint Inhibitors in Colorectal Cancer Therapy.Curr Drug Targets. 2021;22(9):1021-1033. doi: 10.2174/1389450122666210204204415. Curr Drug Targets. 2021. PMID: 33563194 Review.
-
Combined Radiation Therapy and Immune Checkpoint Blockade Therapy for Breast Cancer.Int J Radiat Oncol Biol Phys. 2017 Sep 1;99(1):153-164. doi: 10.1016/j.ijrobp.2017.05.029. Epub 2017 May 26. Int J Radiat Oncol Biol Phys. 2017. PMID: 28816141 Review.
-
Improvement strategy for immune checkpoint blockade: A focus on the combination with immunogenic cell death inducers.Cancer Lett. 2023 May 28;562:216167. doi: 10.1016/j.canlet.2023.216167. Epub 2023 Apr 7. Cancer Lett. 2023. PMID: 37031916 Review.
-
Combining Immunotherapy and Radiotherapy for Cancer Treatment: Current Challenges and Future Directions.Front Pharmacol. 2018 Mar 5;9:185. doi: 10.3389/fphar.2018.00185. eCollection 2018. Front Pharmacol. 2018. PMID: 29556198 Free PMC article. Review.
-
Recent advances in tumor microenvironment-targeted nanomedicine delivery approaches to overcome limitations of immune checkpoint blockade-based immunotherapy.J Control Release. 2021 Apr 10;332:109-126. doi: 10.1016/j.jconrel.2021.02.002. Epub 2021 Feb 8. J Control Release. 2021. PMID: 33571549 Review.
Cited by
-
Exceptional Response to Olaparib and Pembrolizumab for Pancreatic Adenocarcinoma With Germline BRCA1 Mutation and High Tumor Mutation Burden: Case Report and Literature Review.JCO Precis Oncol. 2022 Jan;6:e2100437. doi: 10.1200/PO.21.00437. JCO Precis Oncol. 2022. PMID: 35085003 Free PMC article. Review. No abstract available.
-
Shooting the messenger: a systematic review investigating extracellular vesicle isolation and characterisation methods and their influence on understanding extracellular vesicles-radiotherapy interactions in glioblastoma.BMC Cancer. 2023 Oct 5;23(1):939. doi: 10.1186/s12885-023-11437-6. BMC Cancer. 2023. PMID: 37798728 Free PMC article.
-
Advancements in immunotherapy for colorectal cancer treatment: a comprehensive review of strategies, challenges, and future prospective.Int J Colorectal Dis. 2024 Dec 28;40(1):1. doi: 10.1007/s00384-024-04790-w. Int J Colorectal Dis. 2024. PMID: 39731596 Free PMC article. Review.
-
Leveraging radiotherapy to improve immunotherapy outcomes: rationale, progress and research priorities.Clin Transl Immunology. 2025 Apr 8;14(4):e70030. doi: 10.1002/cti2.70030. eCollection 2025. Clin Transl Immunology. 2025. PMID: 40206193 Free PMC article. Review.
-
Toward Precision Radiotherapy: A Nonlinear Optimization Framework and an Accelerated Machine Learning Algorithm for the Deconvolution of Tumor-Infiltrating Immune Cells.Cells. 2022 Nov 14;11(22):3604. doi: 10.3390/cells11223604. Cells. 2022. PMID: 36429031 Free PMC article.
References
-
- Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA‐approved immune checkpoint inhibitors. Int Immunopharmacol 2018; 62: 29–39. - PubMed
-
- Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Revs Drug Discov 2019; 18: 197–218. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
