Developing a broad-range promoter set for metabolic engineering in the thermotolerant yeast Kluyveromyces marxianus
- PMID: 32995271
- PMCID: PMC7508702
- DOI: 10.1016/j.mec.2020.e00145
Developing a broad-range promoter set for metabolic engineering in the thermotolerant yeast Kluyveromyces marxianus
Erratum in
-
Erratum regarding previously published articles in volumes 9, 10 and 11.Metab Eng Commun. 2021 Oct 28;13:e00186. doi: 10.1016/j.mec.2021.e00186. eCollection 2021 Dec. Metab Eng Commun. 2021. PMID: 34765440 Free PMC article.
Abstract
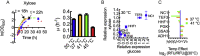
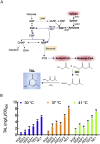
Kluyveromyces marxianus is an emerging host for metabolic engineering. This thermotolerant yeast is the fastest growing eukaryote, has high flux through the TCA cycle, and can metabolize a broad range of C5, C6, and C12 carbon sources. In comparison to the common host Saccharomyces cerevisiae, this non-conventional yeast suffers from a lack of metabolic engineering tools to control gene expression over a wide transcriptional range. To address this issue, we designed a library of 25 native-derived promoters from K. marxanius CBS6556 that spans 87-fold transcriptional strength under glucose metabolism. Six promoters from the library were further characterized in both glucose and xylose as well as across various temperatures from 30 to 45 °C. The temperature study revealed that in most cases EGFP expression decreased with elevating temperature; however, two promoters, P SSA3 and P ADH1 , increased expression above 40 °C in both xylose and glucose. The six-promoter set was also validated in xylose for triacetic acid lactone (TAL) production. By controlling the expression level of heterologous 2-pyrone synthase (2-PS), the specific TAL titer increased over 8-fold at 37 °C. Cultures at 41 °C exhibited a similar TAL biosynthesis capability, while at 30 °C TAL levels were lower. Taken together, these results advance the metabolic engineering tool set in K. marxianus and further develop this new host for chemical biosynthesis.
Keywords: Chemical production; K. marxianus; Non-conventional microbe; Promoter; Thermo-tolerance.
© 2020 The Authors.
Figures


References
-
- Andreu C., Del Olmo M. Whole-cell biocatalysis in seawater: new halotolerant yeast strains for the regio- and stereoselectivity reduction of 1-phenylpropane-1,2-dione in saline-rich media. Chembiochem. 2020;21(11):1621–1628. - PubMed
-
- Benatuil L., Perez J.M., Belk J., Hsieh C.M. An improved yeast transformation method for the generation of very large human antibody libraries. Protein Eng. Des. Sel. 2010;23(4):155–159. - PubMed
-
- Cardenas J., Da Silva N.A. Metabolic engineering of Saccharomyces cerevisiae for the production of triacetic acid lactone. Metab. Eng. 2014;25:194–203. - PubMed
-
- Carlson R. Estimating the biotech sector’s contribution to the US economy. Nat. Biotechnol. 2016;34:247–255. - PubMed
-
- Cernak P., Estrela R., Poddar S., Skerker J.M., Cheng Y.F., Carlson A.K., Chen B., Glynn V.M., Furlan M., Ryan O.W., Donnelly M.K., Arkin A.P., Taylor J.W., Cate J.H.D. Engineering Kluyveromyces marxianus as a robust synthetic biology platform host. Applied and environment Science. 2018;9(5):1–16. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

