Systemic Delivery of AAV- Fdxr Mitigates the Phenotypes of Mitochondrial Disorders in Fdxr Mutant Mice
- PMID: 32995353
- PMCID: PMC7488755
- DOI: 10.1016/j.omtm.2020.05.021
Systemic Delivery of AAV- Fdxr Mitigates the Phenotypes of Mitochondrial Disorders in Fdxr Mutant Mice
Abstract
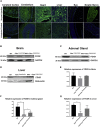
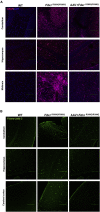
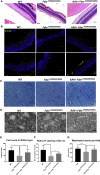
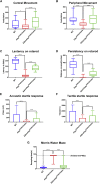
Gene therapy now provides a novel approach for treating inherited monogenetic disorders, including nuclear gene mutations associated with mitochondrial diseases. In this study, we have utilized a mouse model carrying a p.Arg389Gln mutation of the mitochondrial Ferredoxin Reductase gene (Fdxr) and treated them with neurotropic AAV-PHP.B vector loaded with the mouse Fdxr cDNA sequence. We then used immunofluorescence staining and western blot to test the transduction efficiency of this vector. Toluidine blue staining and electronic microscopy were also utilized to assess the morphology of optic and sciatic nerves, and the mitochondrial respiratory chain activity was determined as well. The AAV vector effectively transduced in the central nervous system and peripheral organs, and AAV-Fdxr treatment reversed almost all the symptoms of the mutants (Fdxr R389Q/R389Q ). This therapy also improved the electronic conductivity of the sciatic nerves, prevented optic atrophy, improved mobility, and restored mitochondrial complex function. Most notably, the sensory neuropathy, neurodegeneration, and chronic neuroinflammation in the brain were alleviated. Overall, we present the first demonstration of a potential definitive treatment that significantly improves optic and sciatic nerve atrophy, sensory neuropathy, and mitochondrial dysfunction in FDXR-related mitochondriopathy. Our study provides substantial support for the translation of AAV-based Fdxr gene therapy into clinical applications.
© 2020 The Authors.
Figures




References
-
- Hanukoglu I. Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis. J. Steroid Biochem. Mol. Biol. 1992;43:779–804. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

