In Vitro and In Vivo Amenability to Migalastat in Fabry Disease
- PMID: 32995357
- PMCID: PMC7490640
- DOI: 10.1016/j.omtm.2020.08.012
In Vitro and In Vivo Amenability to Migalastat in Fabry Disease
Abstract
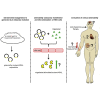
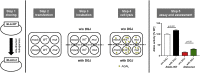
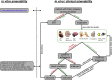
Migalastat (1-deoxygalactonojirimycin) is approved for the treatment of Fabry disease (FD) in patients with an amenable mutation. Currently, there are at least 367 amenable and 711 non-amenable mutations known, based on an in vitro good laboratory practice (GLP) assay. Recent studies demonstrated that in vitro amenability of mutations did not necessarily correspond to in vivo amenability of migalastat-treated patients. This discrepancy might be due to (methodological) limitations of the current GLP-HEK assay. Currently, there are several published comparable cell-based amenability assays, with partially different outcomes for the same tested mutation, leading to concerns in FD-treating physicians. The aim of this review is to elucidate the idea of amenability assays from their beginning, starting with patient-specific primary cells to high-throughput assays based on overexpression. Consequently, we compare methods of current assays, highlighting their similarities, as well as their pros and cons. Finally, we provide a literature-based list of α-galactosidase A mutations, tested by different assays to provide a comprehensive overview of amenable mutations as a good basis for the decision-making by treating physicians. Since in vitro amenability does not always correspond with in vivo amenability, the treating clinician has the responsibility to monitor clinical and laboratory features to verify clinical response.
© 2020 The Author(s).
Figures

References
-
- Zarate Y.A., Hopkin R.J. Fabry’s disease. Lancet. 2008;372:1427–1435. - PubMed
-
- Eng C.M., Guffon N., Wilcox W.R., Germain D.P., Lee P., Waldek S., Caplan L., Linthorst G.E., Desnick R.J., International Collaborative Fabry Disease Study Group Safety and efficacy of recombinant human α-galactosidase A replacement therapy in Fabry’s disease. N. Engl. J. Med. 2001;345:9–16. - PubMed
-
- Schiffmann R., Kopp J.B., Austin H.A., 3rd, Sabnis S., Moore D.F., Weibel T., Balow J.E., Brady R.O. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA. 2001;285:2743–2749. - PubMed
-
- Banikazemi M., Bultas J., Waldek S., Wilcox W.R., Whitley C.B., McDonald M., Finkel R., Packman S., Bichet D.G., Warnock D.G., Desnick R.J., Fabry Disease Clinical Trial Study Group Agalsidase-beta therapy for advanced Fabry disease: a randomized trial. Ann. Intern. Med. 2007;146:77–86. - PubMed
-
- Hughes D.A., Elliott P.M., Shah J., Zuckerman J., Coghlan G., Brookes J., Mehta A.B. Effects of enzyme replacement therapy on the cardiomyopathy of Anderson-Fabry disease: a randomised, double-blind, placebo-controlled clinical trial of agalsidase alfa. Heart. 2008;94:153–158. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

