Doxorubicin Conjugation to Reovirus Improves Oncolytic Efficacy in Triple-Negative Breast Cancer
- PMID: 32995480
- PMCID: PMC7493048
- DOI: 10.1016/j.omto.2020.08.008
Doxorubicin Conjugation to Reovirus Improves Oncolytic Efficacy in Triple-Negative Breast Cancer
Abstract
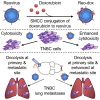
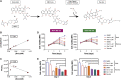
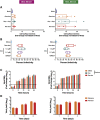
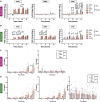
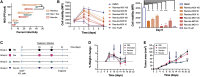
Breast cancer is the second leading cause of cancer-related deaths in women in the United States. The triple-negative breast cancer (TNBC) subtype associates with higher rates of relapse, shorter overall survival, and aggressive metastatic disease. Hormone therapy is ineffective against TNBC, leaving patients with limited therapeutic options. Mammalian orthoreovirus (reovirus) preferentially infects and kills transformed cells, and a genetically engineered reassortant reovirus infects and kills TNBC cells more efficiently than prototypical strains. Reovirus oncolytic efficacy is further augmented by combination with topoisomerase inhibitors, including the frontline chemotherapeutic doxorubicin. However, long-term doxorubicin use correlates with toxicity to healthy tissues. Here, we conjugated doxorubicin to reovirus (reo-dox) to control drug delivery and enhance reovirus-mediated oncolysis. Our data indicate that conjugation does not impair viral biology and enhances reovirus oncolytic capacity in TNBC cells. Reo-dox infection promotes innate immune activation, and crosslinked doxorubicin retains DNA-damaging properties within infected cells. Importantly, reovirus and reo-dox significantly reduce primary TNBC tumor burden in vivo, with greater reduction in metastatic burden after reo-dox inoculation. Together, these data demonstrate that crosslinking chemotherapeutic agents to oncolytic viruses facilitates functional drug delivery to cells targeted by the virus, making it a viable approach for combination therapy against TNBC.
Keywords: DNA damage; cell death; doxorubicin; drug delivery; oncolytics; reovirus; triple-negative breast cancer; virus; virus-host interactions.
© 2020 The Author(s).
Figures



References
-
- American Cancer Society . 2019. Breast Cancer Facts & Figures 2019-2020. (Atlanta: American Cancer Society)
-
- NAACCR.org Available from: https://naaccr.org.
-
- Haffty B.G., Yang Q., Reiss M., Kearney T., Higgins S.A., Weidhaas J., Harris L., Hait W., Toppmeyer D. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J. Clin. Oncol. 2006;24:5652–5657. - PubMed
-
- Dent R., Trudeau M., Pritchard K.I., Hanna W.M., Kahn H.K., Sawka C.A., Lickley L.A., Rawlinson E., Sun P., Narod S.A. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin. Cancer Res. 2007;13:4429–4434. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

