Highly multiplexed, fast and accurate nanopore sequencing for verification of synthetic DNA constructs and sequence libraries
- PMID: 32995546
- PMCID: PMC7445882
- DOI: 10.1093/synbio/ysz025
Highly multiplexed, fast and accurate nanopore sequencing for verification of synthetic DNA constructs and sequence libraries
Abstract
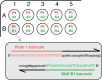
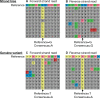
Synthetic biology utilizes the Design-Build-Test-Learn pipeline for the engineering of biological systems. Typically, this requires the construction of specifically designed, large and complex DNA assemblies. The availability of cheap DNA synthesis and automation enables high-throughput assembly approaches, which generates a heavy demand for DNA sequencing to verify correctly assembled constructs. Next-generation sequencing is ideally positioned to perform this task, however with expensive hardware costs and bespoke data analysis requirements few laboratories utilize this technology in-house. Here a workflow for highly multiplexed sequencing is presented, capable of fast and accurate sequence verification of DNA assemblies using nanopore technology. A novel sample barcoding system using polymerase chain reaction is introduced, and sequencing data are analyzed through a bespoke analysis algorithm. Crucially, this algorithm overcomes the problem of high-error rate nanopore data (which typically prevents identification of single nucleotide variants) through statistical analysis of strand bias, permitting accurate sequence analysis with single-base resolution. As an example, 576 constructs (6 × 96 well plates) were processed in a single workflow in 72 h (from Escherichia coli colonies to analyzed data). Given our procedure's low hardware costs and highly multiplexed capability, this provides cost-effective access to powerful DNA sequencing for any laboratory, with applications beyond synthetic biology including directed evolution, single nucleotide polymorphism analysis and gene synthesis.
Keywords: DNA assembly; nanopore sequencing; next-generation sequencing; strand bias; synthetic biology.
© The Author(s) 2019. Published by Oxford University Press.
Figures

References
-
- Paddon C.J., Westfall P.J., Pitera D.J., Benjamin K., Fisher K., McPhee D., Leavell M.D., Tai A., Main A., Eng D. (2013) High-level semi-synthetic production of the potent antimalarial artemisinin. Nature, 496, 528–532. - PubMed
-
- Jang Y.-S., Park J.M., Choi S., Choi Y.J., Seung D.Y., Cho J.H., Lee S.Y. (2012) Engineering of microorganisms for the production of biofuels and perspectives based on systems metabolic engineering approaches. Biotechnol. Adv., 30, 989–1000. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
