Arg-Gly-Asp-modified elastin-like polypeptide regulates cell proliferation and cell cycle proteins via the phosphorylation of Erk and Akt in pancreatic β-cell
- PMID: 32995613
- PMCID: PMC7501433
- DOI: 10.1016/j.heliyon.2020.e04918
Arg-Gly-Asp-modified elastin-like polypeptide regulates cell proliferation and cell cycle proteins via the phosphorylation of Erk and Akt in pancreatic β-cell
Abstract
Objective: Enhancement of β-cell proliferation plays an important role in maintaining β-cell mass and function, and in improving pancreatic β-cell survival before transplantation. Extracellular matrix (ECM) components increase the adhesion and proliferation of β-cells, and the RGD-modified elastin-like polypeptide (RGD-ELP, REP) has been described as a bioactive matrix. In this study, we investigated whether REP could enhance β-cell adhesion and proliferation and elucidated the signaling pathways involved.
Methods: We investigated the effect of REP on cell adhesion, proliferation and insulin secretion via assays using Rin-m and rat islets. Crystal violet, CCK-8, and BrdU assay, FACS, western blot, real time q-PCR analyses and insulin ELISA were examined. To explain the associated mechanisms, phosphorylation of Akt and extracellular signal-regulated kinase (Erk) were measured.
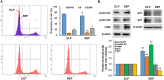
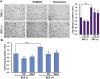
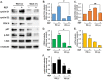
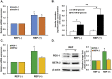
Results: REP more increased the adhesion, proliferation and survival of Rin-m cells compared to elastin-like poly peptide (ELP) without RGD-motif. The enhancement of β-cell proliferation by REP was associated with increased cyclin D1, cyclin D2 and cdk6, and decreased p27 levels. When β-cells were cultured on REP, Erk and the phosphatidylinositol 3-kinase (PI3-kinase) downstream effector, Akt was stimulated. Treatment with the Erk pathway inhibitor and PI3-kinase inhibitor decreased REP-induced β-cell adhesion and proliferation, and regulated REP-induced cell cycle proteins. Additionally, REP increased the mRNA and protein levels of insulin and its transcription factor, PDX-1, and insulin secretion.
Conclusions: Our results demonstrate that the up-regulation of the PI3K/Akt and Erk signaling pathways and the regulation of cell cycle proteins by REP could serve as effective strategies for improving pancreatic β-cell adhesion and proliferation.
Keywords: Biomedical materials; Biotechnology; Cell biology; Cell cycle; Diabetes; Insulin; RGD-modified elastin-like polypeptide; Tissue engineering; β-cell adhesion; β-cell proliferation.
© 2020 The Author(s).
Figures



References
-
- Salomon D., Meda P. Heterogeneity and contact-dependent regulation of hormone secretion by individual B cells. Exp. Cell. Res. 1986;162:507–520. - PubMed
-
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;36:67–74.
-
- Ritz-Laser B., Oberholzer J., Toso C., Brulhar M.C., Zakrzewska K., Ris F., Bucher P., Morel P., Philippe J. Molecular detection of circulating beta-cells after islet transplantation. Diabetes. 2002;51:557–561. - PubMed
-
- Sherry N.A., Kushner J.A., Glandt M., Kitamura T., Brillantes A.M.B., Herold K.C. Effects of autoimmunity and immune therapy on β-Cell turnover in type 1 diabetes. Diabetes. 2006;55:3238–3245. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

