Micellar effects and analytical applications of nitro substitution in 4-Nitro- N-alkyl-1,8-naphthalimide by cysteine derivatives
- PMID: 32995623
- PMCID: PMC7502353
- DOI: 10.1016/j.heliyon.2020.e04938
Micellar effects and analytical applications of nitro substitution in 4-Nitro- N-alkyl-1,8-naphthalimide by cysteine derivatives
Abstract
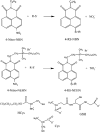
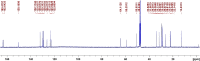
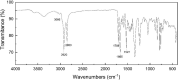
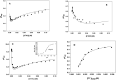
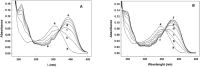
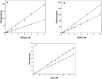
The aromatic nucleophilic substitution reactions of the nitro group of 4-Nitro-N-alkyl-1,8-naphthalimides by thiolate anions produce fluorescent derivatives and their rates are strongly accelerated by micelles of hexadecyltrimethylammonium chloride even at low pH. Acceleration factors of this reactions can reach million-fold. As the products are oxidant-insensible, this reaction allows the determination of SH- containing compounds such as cysteine, glutathione or proteins even in oxidative conditions. Limits of detection are as low as 5 × 10-7 M, ten times lower than the limit for the classic 5,5'-dithiobis-(2-nitrobenzoic) acid method. Moreover, this reaction can be developed at pHs between 6.5 and 7.5 thereby diminishing the rate of spontaneous oxidation of the thiols. In addition, we demonstrated that 4-Nitro-N-alkyl-1,8-naphthalimides can be used to evidence SH groups in peptides, proteins and living cells.
Keywords: Analytical chemistry; Fluorescence assay; Micellar solutions; Organic chemistry; Protein thiols; Thiol levels.
© 2020 The Author(s).
Figures









Similar articles
-
Reactions of papain and of low-molecular-weight thiols with some aromatic disulphides. 2,2'-Dipyridyl disulphide as a convenient active-site titrant for papain even in the presence of other thiols.Biochem J. 1973 May;133(1):67-80. doi: 10.1042/bj1330067. Biochem J. 1973. PMID: 4721623 Free PMC article.
-
Novel chemosensors for detection of glutathione by reduction or substitution of naphthalimide derivatives containing sulfoxide or sulfone substituents.Bioorg Med Chem Lett. 2015 Jan 1;25(1):59-61. doi: 10.1016/j.bmcl.2014.11.028. Epub 2014 Nov 15. Bioorg Med Chem Lett. 2015. PMID: 25465172
-
Naphthalimide Scaffold Provides Versatile Platform for Selective Thiol Sensing and Protein Labeling.ACS Chem Biol. 2016 Apr 15;11(4):1098-105. doi: 10.1021/acschembio.5b00856. Epub 2016 Feb 9. ACS Chem Biol. 2016. PMID: 26813105
-
Heterocyclic Naphthalimides as New Skeleton Structure of Compounds with Increasingly Expanding Relational Medicinal Applications.Curr Top Med Chem. 2016;16(28):3303-3364. doi: 10.2174/1568026616666160506145943. Curr Top Med Chem. 2016. PMID: 27150364 Review.
-
Thiolation and nitrosation of cysteines in biological fluids and cells.Amino Acids. 2003 Dec;25(3-4):323-39. doi: 10.1007/s00726-003-0020-1. Epub 2003 Aug 21. Amino Acids. 2003. PMID: 14661094 Review.
References
-
- Roos G., Foloppe N., Messens J. Understanding the pKa of redox cysteines: the key role of hydrogen bonding. Antioxidants Redox Signal. 2013;18:94–127. - PubMed
-
- Jacob C., Giles G.I., Giles N.M., Sies H. Sulfur and selenium: the role of oxidation state in protein structure and function. Angew. Chem. Int. Ed. 2003;42:4742–4758. - PubMed
-
- Benesch R.E., Benesch R. The acid strength of the -SH group in cysteine and related compounds. J. Am. Chem. Soc. 1955;77:5877–5881.
-
- Danehy J.P., Noel C.J. The relative nucleophilic character of several mercaptans toward ethylene oxide. J. Am. Chem. Soc. 1960;82:2511–2515.
-
- Jung G., Breitmaier E., Voelter W. Dissoziationsgleichgewichte von Glutathion. Eur. J. Biochem. 1972;24:438–445. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

