Usher syndrome: clinical features, molecular genetics and advancing therapeutics
- PMID: 32995707
- PMCID: PMC7502997
- DOI: 10.1177/2515841420952194
Usher syndrome: clinical features, molecular genetics and advancing therapeutics
Abstract
Usher syndrome has three subtypes, each being clinically and genetically heterogeneous characterised by sensorineural hearing loss and retinitis pigmentosa (RP), with or without vestibular dysfunction. It is the most common cause of deaf-blindness worldwide with a prevalence of between 4 and 17 in 100 000. To date, 10 causative genes have been identified for Usher syndrome, with MYO7A accounting for >50% of type 1 and USH2A contributing to approximately 80% of type 2 Usher syndrome. Variants in these genes can also cause non-syndromic RP and deafness. Genotype-phenotype correlations have been described for several of the Usher genes. Hearing loss is managed with hearing aids and cochlear implants, which has made a significant improvement in quality of life for patients. While there is currently no available approved treatment for the RP, various therapeutic strategies are in development or in clinical trials for Usher syndrome, including gene replacement, gene editing, antisense oligonucleotides and small molecule drugs.
Keywords: Usher syndrome; gene therapy; inherited retinal disease; inner ear; photoreceptor; retina; retinitis pigmentosa; sensorineural hearing loss; sensory hair cell.
© The Author(s), 2020.
Conflict of interest statement
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
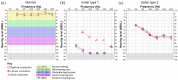

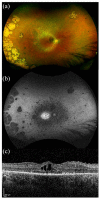

References
-
- Vernon M. Usher’s syndrome – deafness and progressive blindness. J Chronic Dis 1969; 22: 133–151. - PubMed
-
- Boughman JA, Vernon M, Shaver KA. Usher syndrome – definition and estimate of prevalence from two high-risk populations. J Chronic Dis 1983; 36: 595–603. - PubMed
-
- Marazita ML, Ploughman LM, Rawlings B, et al. Genetic epidemiologic studies of early-onset deafness in the United States school-age population. Am J Med Genet 1993; 46: 486–491. - PubMed
-
- Cohen M, Bitner-Glindzicz M, Luxon L. The changing face of Usher syndrome: clinical implications. Int J Audiol 2007; 46: 82–93. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

