Alignment in post-approval changes (PAC) guidelines in emerging countries may increase timely access to vaccines: An illustrative assessment by manufacturers
- PMID: 32995745
- PMCID: PMC7516132
- DOI: 10.1016/j.jvacx.2020.100075
Alignment in post-approval changes (PAC) guidelines in emerging countries may increase timely access to vaccines: An illustrative assessment by manufacturers
Abstract
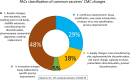
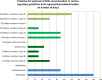
A comparison of the regulations and guidelines from 33 countries, across different regions, on the requirements and procedures for the management of chemical, manufacturing and control (CMC) changes for vaccines, also known as post- approval changes (PACs), reveals significant variability and lack of predictability of timelines for regulatory review and approval. These shortcomings imply that multiple data packages have to be prepared for submission to different authorities, generating a complex regulatory environment. Moreover, the timelines for approval by individual national regulatory authorities are variable, which results in manufacturers keeping various stocks of vaccines produced in accordance with the various approved specifications and procedures, in the different countries. This can seriously affect timely availability of vaccine in those countries. The World Health Organization (WHO) guidelines on procedures and data requirements for changes to approved vaccines provide a consensual framework for alignment, but are still underused. Reliance on both the review and approval by the regulatory authority in the country of manufacturing, or on the review performed by other national regulatory authorities, recognized by WHO as stringent, or on WHO prequalification dossier, offer alternative ways forward. These and other options to improve the management of post-approval changes during the product lifecycle of vaccines are discussed in this report, and aimed at improving guidelines alignment and regulatory convergence to advance immunization equity and coverage.
Keywords: Changes classification; Post-approval changes; Regulatory convergence; Regulatory science; Vaccine access; Variations guidelines.
© 2020 DCVMN International.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Dellepiane N, Pagliusi S, Regulatory Experts Working Group. Challenges for the registration of vaccines in emerging countries: Differences in dossier requirements, application and evaluation processes. N. Dellepiane, S. Pagliusi and Registration Experts Working Group. Vaccine 2018; 36(24): 3389-3396. Cf. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6278877/. - PMC - PubMed
-
- Dellepiane N, Pagliusi S, Regulatory Experts Working Group. Opportunities for improving access to vaccines in emerging countries through efficient and aligned registration procedures: An industry perspective. Vaccine 2019; 37: 2982-2989. Cf. https://pubmed.ncbi.nlm.nih.gov/31027928/. - PubMed
-
- John F. Enders, Ph.D.†, Samuel L. Katz, M.D. et al. Studies on an Attenuated Measles-Virus Vaccine — Development and Preparation of the Vaccine: Technics for Assay of Effects of Vaccination. N Engl J Med 1960; 263:153-159. Cf. https://pubmed.ncbi.nlm.nih.gov/14404830/. - PubMed
-
- Theiler M, Smith HH. The use of yellow fever virus modified by in vitro cultivation for human immunization. J. Exp. Med 1937; 65:787-800. Cf. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2133527/. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

