This is a preprint.
Cyclooxgenase-2 is induced by SARS-CoV-2 infection but does not affect viral entry or replication
- PMID: 32995789
- PMCID: PMC7523115
- DOI: 10.1101/2020.09.24.312769
Cyclooxgenase-2 is induced by SARS-CoV-2 infection but does not affect viral entry or replication
Abstract
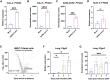
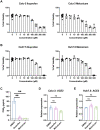
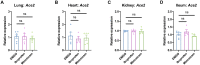
Identifying drugs that regulate severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and its symptoms has been a pressing area of investigation during the coronavirus disease 2019 (COVID-19) pandemic. Nonsteroidal anti-inflammatory drugs (NSAIDs), which are frequently used for the relief of pain and inflammation, could modulate both SARS-CoV-2 infection and the host response to the virus. NSAIDs inhibit the enzymes cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2), which mediate the production of prostaglandins (PGs). PGE2, one of the most abundant PGs, has diverse biological roles in homeostasis and inflammatory responses. Previous studies have shown that NSAID treatment or inhibition of PGE2 receptor signaling leads to upregulation of angiotensin-converting enzyme 2 (ACE2), the cell entry receptor for SARS-CoV-2, thus raising concerns that NSAIDs could increase susceptibility to infection. COX/PGE2 signaling has also been shown to regulate the replication of many viruses, but it is not yet known whether it plays a role in SARS-CoV-2 replication. The purpose of this study was to dissect the effect of NSAIDs on COVID-19 in terms of SARS-CoV-2 entry and replication. We found that SARS-CoV-2 infection induced COX-2 upregulation in diverse human cell culture and mouse systems. However, suppression of COX-2/PGE2 signaling by two commonly used NSAIDs, ibuprofen and meloxicam, had no effect on ACE2 expression, viral entry, or viral replication. Our findings suggest that COX-2 signaling driven by SARS-CoV-2 may instead play a role in regulating the lung inflammation and injury observed in COVID-19 patients.
Conflict of interest statement
Competing interests None of the authors declare competing interests related to this manuscript.
Figures

References
-
- Day M. 2020. Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ 368. - PubMed
-
- Powis S. 2020. Novel Coronavirus - Anti-inflammatory medications. Medicines and Healthcare products Regulatory Agency.
-
- Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, Chen H-D, Chen J, Luo Y, Guo H, Jiang R-D, Liu M-Q, Chen Y, Shen X-R, Wang X, Zheng X-S, Zhao K, Chen Q-J, Deng F, Liu L-L, Yan B, Zhan F-X, Wang Y-Y, Xiao G-F, Shi Z-L. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. 7798. Nature 579:270–273. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
