SGLT2 Inhibitors: Slowing of Chronic Kidney Disease Progression in Type 2 Diabetes
- PMID: 32996085
- PMCID: PMC7524028
- DOI: 10.1007/s13300-020-00930-x
SGLT2 Inhibitors: Slowing of Chronic Kidney Disease Progression in Type 2 Diabetes
Abstract
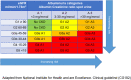
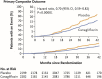
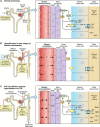
Diabetic kidney disease (DKD) is a topic of increasing concern among clinicians involved in the management of type 2 diabetes mellitus (T2DM). It is a progressive and costly complication associated with increased risk of adverse cardiovascular (CV) and renal outcomes and mortality. Ongoing monitoring of the estimated glomerular filtration (eGFR) rate alongside the urine albumin:creatinine ratio (ACR) is recommended during regular T2DM reviews to enable a prompt DKD diagnosis or to assess disease progression, providing an understanding of adverse risk for each individual. Many people with DKD will progress to end-stage kidney disease (ESKD), requiring renal replacement therapy (RRT), typically haemodialysis or kidney transplantation. A range of lifestyle and pharmacological interventions is recommended to help lower CV risk, slow the advancement of DKD and prevent or delay the need for RRT. Emerging evidence concerning sodium-glucose co-transporter-2 inhibitor (SGLT2i) agents suggests a role for these medicines in slowing eGFR decline, enabling regression of albuminuria and reducing progression to ESKD. Improvements in renal end points observed in SGLT2i CV outcome trials (CVOTs) highlighted the possible impact of these agents in the management of DKD. Data from the canagliflozin CREDENCE trial (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) have since demonstrated the effectiveness of this medicine in reducing the risk of kidney failure and CV events in a population comprising individuals with T2DM and renal disease. CREDENCE was the first SGLT2i study to examine renal outcomes as the primary end point. Real-world studies have reaffirmed these outcomes in routine clinical practice. This article summarises the evidence regarding the use of SGLT2i medicines in slowing the progression of DKD and examines the possible mechanisms underpinning the renoprotective effects of these agents. The relevant national and international guidance for monitoring and treatment of DKD is also highlighted to help clinicians working to support this vulnerable group.
Keywords: Chronic kidney disease; Diabetic kidney disease; End-stage kidney disease; Kidney failure; Oral glucose-lowering medicines; SGLT2 inhibitors; Type 2 diabetes.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

