Epicutaneous immunotherapy protects cashew-sensitized mice from anaphylaxis
- PMID: 32996148
- PMCID: PMC8246921
- DOI: 10.1111/all.14605
Epicutaneous immunotherapy protects cashew-sensitized mice from anaphylaxis
Abstract
Background: The prevalence of tree nut allergy has increased worldwide, and cashew has become one of the most common food allergens. More critically, cashew allergy is frequently associated with severe anaphylaxis. Despite the high medical need, no approved treatment is available and strict avoidance and preparedness for prompt treatment of allergic reactions are considered dual standard of care. In the meantime, Phase III study results suggest investigational epicutaneous immunotherapy (EPIT) may be a relevant and safe treatment for peanut allergy and may improve the quality of life for many peanut allergic children.
Objective: We aimed to evaluate the capacity of EPIT to provide protection against cashew-induced anaphylaxis in a relevant mouse model.
Methods: The efficacy of EPIT was evaluated by applying patches containing cashew allergens to cashew-sensitized mice. As negative control, sham mice received patches containing excipient. Following treatment, mice were challenged orally to cashew and anaphylactic symptoms, as well as plasmatic levels of mast-cell proteases (mMCP)-1/7, were quantified.
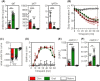
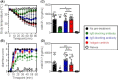
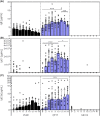
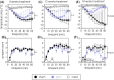
Results: Of 16 weeks of EPIT significantly protects against anaphylaxis by promoting a faster recovery of challenged mice. This protection was characterized by a significant reduction of temperature drop and clinical symptoms, 60 minutes after challenge. This was associated with a decrease in mast-cell reactivity as attested by the reduction of mMCP-1/7 in plasma, suggesting that EPIT specifically decrease IgE-mediated anaphylaxis.
Conclusion: We demonstrate that EPIT markedly reduced IgE-mediated allergic reactions in a mouse model of cashew allergy, which suggests that EPIT may be a relevant approach to treating cashew allergy.
Keywords: Viaskin; anaphylaxis mouse model; cashew allergy; epicutaneous immunotherapy.
© 2020 The Authors. Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
Benjamin Pelletier, Camille Plaquet, Jean‐Louis Labernardière, Mélanie Ligouis, Dr. Dioszeghy, Dr. Wavrin, Dr. Matthews, Dr. Sampson, and Dr. Hervé report personal fees from DBV Technologies, during the conduct of the study; personal fees from DBV Technologies, outside the submitted work. Audrey Perrin, Noémie Assoun, Nathalie Oreal, Laetitia Gaulme, Adeline Bouzereau, and Dr. Porcheray report personal fees from DBV Technologies, during the conduct of the study.
Figures


Similar articles
-
Epicutaneous immunotherapy induces gastrointestinal LAP+ regulatory T cells and prevents food-induced anaphylaxis.J Allergy Clin Immunol. 2017 Jan;139(1):189-201.e4. doi: 10.1016/j.jaci.2016.03.057. Epub 2016 Jun 11. J Allergy Clin Immunol. 2017. PMID: 27417020 Free PMC article.
-
Specific epicutaneous immunotherapy prevents sensitization to new allergens in a murine model.J Allergy Clin Immunol. 2015 Jun;135(6):1546-57.e4. doi: 10.1016/j.jaci.2014.11.028. Epub 2015 Jan 9. J Allergy Clin Immunol. 2015. PMID: 25583102
-
Targeted allergen-specific immunotherapy within the skin improves allergen delivery to induce desensitization to peanut.Immunotherapy. 2022 May;14(7):539-552. doi: 10.2217/imt-2021-0206. Epub 2022 Feb 24. Immunotherapy. 2022. PMID: 35196877 Free PMC article.
-
Cashew Nut Allergy: Clinical Relevance and Allergen Characterisation.Clin Rev Allergy Immunol. 2019 Aug;57(1):1-22. doi: 10.1007/s12016-016-8580-5. Clin Rev Allergy Immunol. 2019. PMID: 27585580 Review.
-
Epicutaneous peanut patch device for the treatment of peanut allergy.Expert Rev Clin Immunol. 2019 May;15(5):449-460. doi: 10.1080/1744666X.2019.1593138. Epub 2019 Mar 27. Expert Rev Clin Immunol. 2019. PMID: 30864861 Review.
Cited by
-
Mechanisms and biomarkers of successful allergen-specific immunotherapy.Asia Pac Allergy. 2022 Oct 31;12(4):e45. doi: 10.5415/apallergy.2022.12.e45. eCollection 2022 Oct. Asia Pac Allergy. 2022. PMID: 36452016 Free PMC article. Review.
-
Advancing immunotherapy using biomaterials to control tissue, cellular, and molecular level immune signaling in skin.Adv Drug Deliv Rev. 2024 Jun;209:115315. doi: 10.1016/j.addr.2024.115315. Epub 2024 Apr 25. Adv Drug Deliv Rev. 2024. PMID: 38670230 Free PMC article. Review.
-
Recent advances in epicutaneous immunotherapy and potential applications in food allergy.Front Allergy. 2023 Oct 27;4:1290003. doi: 10.3389/falgy.2023.1290003. eCollection 2023. Front Allergy. 2023. PMID: 37965375 Free PMC article. Review.
-
Microarray Patch Based Transdermal Allergen Immunotherapy Prevents Gliadin-induced Anaphylaxis in a Murine Model.Allergy Asthma Immunol Res. 2025 May;17(3):330-348. doi: 10.4168/aair.2025.17.3.330. Allergy Asthma Immunol Res. 2025. PMID: 40414810 Free PMC article.
-
Anti-IgE therapy versus allergen-specific immunotherapy for food allergy: weighing the pros and cons.Front Immunol. 2025 Jul 22;16:1617153. doi: 10.3389/fimmu.2025.1617153. eCollection 2025. Front Immunol. 2025. PMID: 40766311 Free PMC article. Review.
References
-
- Mendes C, Costa J, Vicente AA, Oliveira MBPP, Mafra I. Cashew Nut Allergy: Clinical Relevance and Allergen Characterisation. Clin Rev Allergy Immunol. 2019;57(1):1‐22. - PubMed
-
- Johnson J, Malinovschi A, Alving K, Lidholm J, Borres MP, Nordvall L. Ten‐year review reveals changing trends and severity of allergic reactions to nuts and other foods. Acta Paediatr Int. J Paediatr. 2014;103(8);862. - PubMed
-
- McWilliam V, Koplin J, Lodge C, Tang M, Dharmage S, Allen K. The Prevalence of Tree Nut Allergy: A Systematic Review. Curr Allergy Asthma Rep. 2015;15:54. - PubMed
-
- Crealey M, Alamin S, Tormey V, Moylett E. Clinical presentation of cashew nut allergy in a paediatric cohort attending an allergy clinic in the West of Ireland. Ir J Med Sci. 2019;188:219‐222. - PubMed
-
- Clark AT, Anagnostou K, Ewan PW. Cashew nut causes more severe reactions than peanut: Case‐matched comparison in 141 children. Allergy. 2007;62(8):913‐916. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

