H2S attenuates oxidative stress via Nrf2/NF-κB signaling to regulate restenosis after percutaneous transluminal angioplasty
- PMID: 32996350
- PMCID: PMC7871122
- DOI: 10.1177/1535370220961038
H2S attenuates oxidative stress via Nrf2/NF-κB signaling to regulate restenosis after percutaneous transluminal angioplasty
Abstract
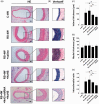
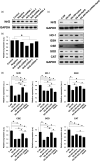
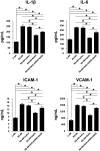
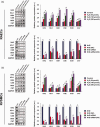
Restenosis after angioplasty of peripheral arteries is a clinical problem involving oxidative stress. Hydrogen sulfide (H2S) participates in oxidative stress regulation and activates nuclear factor erythroid 2-related factor 2 (Nrf2). This study investigated the effect of H2S and Nrf2 on restenosis-induced arterial injury. Using an in vivo rat model of restenosis, we investigated whether H2S inhibits restenosis after percutaneous transluminal angioplasty (PTA) and the oxidative stress-related mechanisms implicated therein. The involvement of Nrf2 was explored using Nrf2-shRNA. Neointimal formation and the deposition of elastic fibers were assessed histologically. Inflammatory cytokine secretion and the expression of proteins associated with oxidative stress and inflammation were evaluated. The artery of rats subjected to restenosis showed increased arterial intimal thickness, with prominent elastic fiber deposition. Sodium hydrosulfide (NaHS), an H2S donor, counteracted these changes in vivo. Restenosis caused a decrease in anti-oxidative stress signaling. This phenomenon was inhibited by NaHS, but Nrf2-shRNA counteracted the effects of NaHS. In terms of inflammation, inflammatory cytokines were upregulated, whereas NaHS suppressed the induced inflammatory reaction. Similarly, Nrf2 downregulation blocked the effect of NaHS. In vitro studies using aortic endothelial and vascular smooth muscle cells isolated from experimental animals showed consistent results as those of in vivo studies, and the participation of the nuclear factor-kappa B signaling pathway was demonstrated. Collectively, H2S played a role in regulating post-PTA restenosis by alleviating oxidative stress, modulating anti-oxidant defense, and targeting Nrf2-related pathways via nuclear factor-kappa B signaling.
Keywords: Hydrogen sulfide; anti-oxidant defense; inflammation; neointimal hyperplasia; nuclear factor erythroid 2-related factor 2.
Conflict of interest statement
Figures



Similar articles
-
Protective effect of a hydrogen sulfide donor on balloon injury-induced restenosis via the Nrf2/HIF-1α signaling pathway.Int J Mol Med. 2019 Mar;43(3):1299-1310. doi: 10.3892/ijmm.2019.4076. Epub 2019 Jan 23. Int J Mol Med. 2019. PMID: 30747216 Free PMC article.
-
Hydrogen sulfide (H2S) attenuates uranium-induced acute nephrotoxicity through oxidative stress and inflammatory response via Nrf2-NF-κB pathways.Chem Biol Interact. 2015 Dec 5;242:353-62. doi: 10.1016/j.cbi.2015.10.021. Epub 2015 Oct 31. Chem Biol Interact. 2015. PMID: 26523793
-
Effect of hydrogen sulfide on restenosis of peripheral arteries after angioplasty.Mol Med Rep. 2012 Jun;5(6):1497-502. doi: 10.3892/mmr.2012.853. Epub 2012 Apr 2. Mol Med Rep. 2012. PMID: 22470131
-
Hydrogen sulfide protects the endometrium in a rat model of type 1 diabetes via modulation of PPARγ/mTOR and Nrf-2/NF-κb pathways.Arch Physiol Biochem. 2024 Dec;130(6):909-920. doi: 10.1080/13813455.2024.2347239. Epub 2024 Apr 29. Arch Physiol Biochem. 2024. PMID: 38685691 Review.
-
Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis.Biochem Pharmacol. 2008 Dec 1;76(11):1485-9. doi: 10.1016/j.bcp.2008.07.017. Epub 2008 Jul 23. Biochem Pharmacol. 2008. PMID: 18694732 Free PMC article. Review.
Cited by
-
H2S Prodrug, SG-1002, Protects against Myocardial Oxidative Damage and Hypertrophy In Vitro via Induction of Cystathionine β-Synthase and Antioxidant Proteins.Biomedicines. 2023 Feb 18;11(2):612. doi: 10.3390/biomedicines11020612. Biomedicines. 2023. PMID: 36831146 Free PMC article.
-
Hydrogen Sulfide as a Potential Therapy for Heart Failure-Past, Present, and Future.Antioxidants (Basel). 2021 Mar 19;10(3):485. doi: 10.3390/antiox10030485. Antioxidants (Basel). 2021. PMID: 33808673 Free PMC article. Review.
-
Immune mechanism of gut microbiota and its metabolites in the occurrence and development of cardiovascular diseases.Front Microbiol. 2022 Dec 14;13:1034537. doi: 10.3389/fmicb.2022.1034537. eCollection 2022. Front Microbiol. 2022. PMID: 36590426 Free PMC article. Review.
-
Mechanistic Intimate Insights into the Role of Hydrogen Sulfide in Alzheimer's Disease: A Recent Systematic Review.Int J Mol Sci. 2023 Oct 23;24(20):15481. doi: 10.3390/ijms242015481. Int J Mol Sci. 2023. PMID: 37895161 Free PMC article. Review.
-
Causal relationship between mitochondrial proteins and risks of aortic aneurysms and aortic dissection: a Mendelian randomization study.J Cardiothorac Surg. 2025 Apr 5;20(1):181. doi: 10.1186/s13019-025-03389-8. J Cardiothorac Surg. 2025. PMID: 40186305 Free PMC article.
References
-
- Zhou X, Dong J, Zhang L, Liu J, Dong X, Yang Q, Liu F, Liao L. Hyperglycemia has no effect on development of restenosis after percutaneous transluminal angioplasty (PTA) in a diabetic rabbit model. J Endocrinol 2015; 224:119–25 - PubMed
-
- Hajibandeh S, Hajibandeh S, Antoniou SA, Torella F, Antoniou GA. Treatment strategies for in-stent restenosis in peripheral arterial disease: a systematic review. Interact Cardiovasc Thorac Surg 2019; 28:253–61 - PubMed
-
- Fritz P, Stein U, Hasslacher C, Zierhut D, Wannenmacher M, Pritsch M. External beam radiotherapy fails to prevent restenosis after iliac or femoropopliteal percutaneous transluminal angioplasty: results of a prospective randomized double-blind study. Int J Radiat Oncol Biol Phys 2004; 59:815–21 - PubMed
-
- Wang X, Li J, Zhang H, Zhang Y. Evaluation of the small intestinal submucosa covered stent in preventing restenosis after percutaneous transluminal angioplasty in the swine. Eur J Radiol 2012; 81:e281. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

