The Effect of Dose Escalation on the Cost-Effectiveness of Etanercept and Adalimumab with Methotrexate Among Patients with Moderate to Severe Rheumatoid Arthritis
- PMID: 32996384
- PMCID: PMC10391279
- DOI: 10.18553/jmcp.2020.26.10.1236
The Effect of Dose Escalation on the Cost-Effectiveness of Etanercept and Adalimumab with Methotrexate Among Patients with Moderate to Severe Rheumatoid Arthritis
Abstract
Background: Patients with moderate to severe rheumatoid arthritis (RA) occasionally increase their doses of tumor necrosis factor (TNF) inhibitors, especially the monoclonal antibody origin drugs such as adalimumab and infliximab, after inadequate response to the initial dose. Previous studies have evaluated the cost-effectiveness of various sequences of treatment for RA in the United States but have not considered the effect of dose escalation.
Objective: To assess the cost-effectiveness of etanercept and adalimumab by incorporating the effect of dose escalation in moderate to severe RA patients.
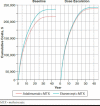
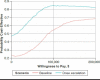
Methods: We adapted the open-source Innovation and Value Initiative - Rheumatoid Arthritis model, version 1.0 to separately simulate the magnitude and time to dose escalation among RA patients taking adalimumab plus methotrexate or etanercept plus methotrexate from a societal perspective and lifetime horizon. An important assumption in the model was that dose escalation would increase treatment costs through its effect on the number of doses but would have no effect on effectiveness. We estimated the dose escalation parameters using the IBM MarketScan Commercial and Medicare Supplemental Databases. We fit competing parametric survival models to model time to dose escalation and used model diagnostics to compare the fit of the competing models. We measured the magnitude of dose escalation as the percentage increase in the number of doses conditional on dose escalation. Finally, we used the parameterized model to simulate treatment sequences beginning with a TNF inhibitor (adalimumab, etanercept) followed by nonbiologic treatment.
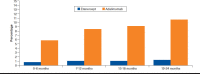
Results: In baseline models without dose escalation, the incremental cost per quality-adjusted life-year of the etanercept treatment sequence relative to the adalimumab treatment sequence was $85,593. Incorporating dose escalation increased treatment costs for each sequence, but costs increased more with adalimumab, lowering the incremental cost-effectiveness ratio to $9,001. At willingness-to-pay levels of $100,000, the etanercept sequence was more cost-effective compared with the adalimumab sequence, with probability 0.55 and 0.85 in models with and without dose escalation, respectively.
Conclusions: Dose escalation has important effects on cost-effectiveness and should be considered when comparing biologic medications for the treatment of RA.
Disclosures: Funding for this study was contributed by Amgen. When this work was conducted, Incerti and Jansen were employees of Precision Health Economics, which received financial support from Amgen. Maksabedian Hernandez, Collier, Gharaibeh, and Stolshek were employees and stockholders of Amgen, and Tkacz and Moore-Schiltz were employees of IBM Watson Health, which received financial support from Amgen. Some of the results of this work were previously presented as a poster at the 2019 AMCP Managed Care & Specialty Pharmacy Annual Meeting, March 25-28, 2019, in San Diego, CA.
Conflict of interest statement
Funding for this study was contributed by Amgen. When this work was conducted, Incerti and Jansen were employees of Precision Health Economics, which received financial support from Amgen. Maksabedian Hernandez, Collier, Gharaibeh, and Stolshek were employees and stockholders of Amgen, and Tkacz and Moore-Schiltz were employees of IBM Watson Health, which received financial support from Amgen.
Some of the results of this work were previously presented as a poster at the 2019 AMCP Managed Care & Specialty Pharmacy Annual Meeting, March 25-28, 2019, in San Diego, CA
Figures
Similar articles
-
Treatment Patterns and Costs in Biologic DMARD-Naive Patients with Rheumatoid Arthritis Initiating Etanercept or Adalimumab with or Without Methotrexate.J Manag Care Spec Pharm. 2020 Mar;26(3):285-294. doi: 10.18553/jmcp.2020.26.3.285. J Manag Care Spec Pharm. 2020. PMID: 32105179 Free PMC article.
-
Effectiveness and Costs Among Rheumatoid Arthritis Patients Treated with Targeted Immunomodulators Using Real-World U.S. Data.J Manag Care Spec Pharm. 2020 Aug;26(8):1039-1049. doi: 10.18553/jmcp.2020.26.8.1039. J Manag Care Spec Pharm. 2020. PMID: 32715967 Free PMC article.
-
Cost-utility analysis of certolizumab pegol in combination with methotrexate in patients with moderate-to-severe active rheumatoid arthritis in Greece.Rheumatol Int. 2017 Sep;37(9):1441-1452. doi: 10.1007/s00296-017-3736-z. Epub 2017 May 18. Rheumatol Int. 2017. PMID: 28523420
-
Review of eight pharmacoeconomic studies of the value of biologic DMARDs (adalimumab, etanercept, and infliximab) in the management of rheumatoid arthritis.J Manag Care Pharm. 2006 Sep;12(7):555-69. doi: 10.18553/jmcp.2006.12.7.555. J Manag Care Pharm. 2006. PMID: 16981801 Free PMC article. Review.
-
Cost-effectiveness of TNF-alpha-blocking agents in the treatment of rheumatoid arthritis.Expert Opin Pharmacother. 2004 Sep;5(9):1881-6. doi: 10.1517/14656566.5.9.1881. Expert Opin Pharmacother. 2004. PMID: 15330726 Review.
Cited by
-
Four Aspects Affecting Health Economic Decision Models and Their Validation.Pharmacoeconomics. 2022 Mar;40(3):241-248. doi: 10.1007/s40273-021-01110-w. Epub 2021 Dec 16. Pharmacoeconomics. 2022. PMID: 34913142
-
Cost-Effectiveness Analysis of Biopharmaceuticals for Treating Rheumatoid Arthritis: Infliximab, Adalimumab, and Etanercept.Biomed Res Int. 2021 Nov 28;2021:4450162. doi: 10.1155/2021/4450162. eCollection 2021. Biomed Res Int. 2021. PMID: 34877355 Free PMC article.
References
-
- Helmick CG, Felson DT, Lawrence RC, et al. . Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15-25. - PubMed
-
- Birnbaum H, Pike C, Kaufman R, Marynchenko M, Kidolezi Y, Cifaldi M. Societal cost of rheumatoid arthritis patients in the U.S. Curr Med Res Opin. 2010; 26(1):77-90. - PubMed
-
- Lundkvist J, Kastang F, Kobelt G. The burden of rheumatoid arthritis and access to treatment: health burden and costs. Eur J Health Econ. 2008;8(Suppl 2):S49-60. - PubMed
-
- Singh JA, Saag KG, Bridges SL Jr, et al. . 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68(1):1-26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical