Antinociceptive modulation by the adhesion GPCR CIRL promotes mechanosensory signal discrimination
- PMID: 32996461
- PMCID: PMC7546736
- DOI: 10.7554/eLife.56738
Antinociceptive modulation by the adhesion GPCR CIRL promotes mechanosensory signal discrimination
Abstract
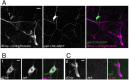
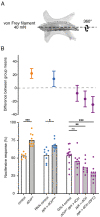
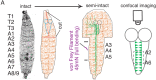
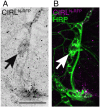
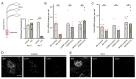
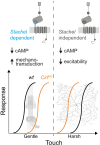
Adhesion-type GPCRs (aGPCRs) participate in a vast range of physiological processes. Their frequent association with mechanosensitive functions suggests that processing of mechanical stimuli may be a common feature of this receptor family. Previously, we reported that the Drosophila aGPCR CIRL sensitizes sensory responses to gentle touch and sound by amplifying signal transduction in low-threshold mechanoreceptors (Scholz et al., 2017). Here, we show that Cirl is also expressed in high-threshold mechanical nociceptors where it adjusts nocifensive behaviour under physiological and pathological conditions. Optogenetic in vivo experiments indicate that CIRL lowers cAMP levels in both mechanosensory submodalities. However, contrasting its role in touch-sensitive neurons, CIRL dampens the response of nociceptors to mechanical stimulation. Consistent with this finding, rat nociceptors display decreased Cirl1 expression during allodynia. Thus, cAMP-downregulation by CIRL exerts opposing effects on low-threshold mechanosensors and high-threshold nociceptors. This intriguing bipolar action facilitates the separation of mechanosensory signals carrying different physiological information.
Keywords: D. melanogaster; adhesion-GPCR; mechanosensory physiology; neuroscience; nociception; rat.
© 2020, Dannhäuser et al.
Conflict of interest statement
SD, TL, CH, MS, JC, NE, DS, SS, DP, MP, TL, PS, HR, RK No competing interests declared
Figures



References
-
- Boiko N, Medrano G, Montano E, Jiang N, Williams CR, Madungwe NB, Bopassa JC, Kim CC, Parrish JZ, Hargreaves KM, Stockand JD, Eaton BA. TrpA1 activation in peripheral sensory neurons underlies the ionic basis of pain hypersensitivity in response to Vinca alkaloids. PLOS ONE. 2017;12:e0186888. doi: 10.1371/journal.pone.0186888. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

