Randomized Clinical Trial of a Legacy Intervention for Quality of Life in Children with Advanced Cancer
- PMID: 32996842
- PMCID: PMC8064943
- DOI: 10.1089/jpm.2020.0139
Randomized Clinical Trial of a Legacy Intervention for Quality of Life in Children with Advanced Cancer
Abstract
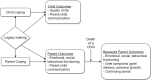
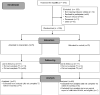
Background: Legacy-making (actions/behaviors aimed at being remembered) may be a significant component for quality of life (QOL) during advanced illness and end of life. Although legacy interventions have been tested in adults, the impact of legacy activities on QOL for children has yet to be clearly defined. Objective: This study examined the impact of our newly developed web-based legacy intervention on dimensions of QOL among children (7-17 years old) with advanced cancer. Design: This single-site randomized clinical trial (RCT) used a two-group waitlist control design. The legacy intervention guided children to create digital storyboards by directing them to answer legacy questions about themselves (personal characteristics, things they like to do, and connectedness with others) and upload photographs, video, and music. Setting/Subjects: Facebook advertisements recruited children (ages 7-17) with relapsed/refractory cancer and their parents from the United States. Child-parent dyads (N = 150) were randomized to the intervention or usual care group, and 97 dyads were included for analysis. Measurements: Children and parents completed the PedsQL Cancer Module preintervention (T1) and post-intervention (T2). Results: Although not statistically significant, legacy-making demonstrated small effects in child procedural anxiety and perceived physical appearance (Cohen's d 0.35-0.28) compared to the wait-list control group. Conclusions: This study contributes important discoveries, including support for the feasibility of a RCT web-based legacy intervention for children with advanced cancer. We did not find convincing evidence supporting the hypothesis that legacy-making improved child dimensions of QOL across time. Overall, this is a null study that warrants discussion on possible reasons for limited findings. Future legacy intervention research is needed using qualitative and quantitative methods, as well as child and parent reports, to determine how such services may improve dimensions of QOL for pediatric palliative care populations. ClinicalTrials.gov number NCT04059393.
Keywords: Internet-based intervention; pediatric cancer; pediatric palliative care; quality of life.
Conflict of interest statement
No competing financial interests exist.
Figures
References
-
- Siegel RL, Miller KD, Jemal A: Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30 - PubMed
-
- Wolfe J, Grier HE, Klar N, et al. : Symptoms and suffering at the end of life in children with cancer. N Engl J Med 2000;342:326–333 - PubMed
-
- Olagunju AT, Sarimiye FO, Olagunju TO, et al. : Child's symptom burden and depressive symptoms among caregivers of children with cancers: An argument for early integration of pediatric palliative care. Ann Palliat Med 2016;5:157–165 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical