In vivo and in vitro activation of dormant primordial follicles by EGF treatment in mouse and human
- PMID: 32997412
- PMCID: PMC7520080
- DOI: 10.1002/ctm2.182
In vivo and in vitro activation of dormant primordial follicles by EGF treatment in mouse and human
Abstract
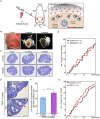
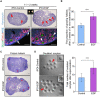
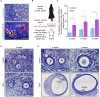
In the mammalian ovaries, dormant primordial follicles represent the reproductive reserve of individual females. Recently, stimulating the activation of primordial follicles in vitro has been practiced, making the utilization of those dormant follicles to treat female infertility possible. However, there are still lacks of effective upstream molecule and strategy to elevate follicle activation in vivo. In the current study, we revealed that growth factor EGF improved a transiently primordial follicle activation in mice by elevating the CDC42-PI3K signaling activity, and EGF treatment also improved the activation and development of human follicles in ovarian cortical pieces. Using a liquid-solid phase transition bio-gel as a carrier, an efficient in vivo activation system was established by ovarian topical EGF administration to living mice. We found that EGF treatment led to an increase of primordial follicle activation in short time but had no effect on long-term fertility in females. By establishing an inducible premature ovarian insufficiency (POI) mouse model through selectively ablating growing follicles in Zp3-Cre;iDTR mice, we further revealed that our in vivo EGF treatment system improved primordial follicle activation and ovulation of POI ovaries significantly. Taken together, our results revealed that in situ ovarian EGF administration could improve the activation of primordial follicles in living animals, and manipulating activation and development of primordial follicles in vivo might be an efficient approach to improve reproductive health in women.
Keywords: EGF; in vivo activation; noninvasive administration; premature ovarian insufficiency; primordial follicle activation.
© 2020 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
Jiawei Zhang, Lei Yan, Yibo Wang, Shuo Zhang, Xueqiang Xu, Yanli Dai, Shidou Zhao, Zhen Li, Yan Zhang, Guoliang Xia, Yingying Qin, and Hua Zhang declare that they have no conflict of interest.
Figures


References
-
- Eppig JJ. Intercommunication between mammalian oocytes and companion somatic cells. Bioessays. 1991;13:569‐574. - PubMed
-
- Adhikari D, Liu K. Molecular Mechanisms Underlying the Activation of Mammalian Primordial Follicles. Endocr Rev. 2009;30:438‐464. - PubMed
-
- Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968;17:555‐557. - PubMed
-
- Gougeon A. Regulation of Ovarian Follicular Development in Primates: facts and Hypotheses. Endocr Rev. 1996;17:121‐155. - PubMed
-
- Mcgee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200‐214. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
