Bacterial and viral infections and related inflammatory responses in chronic obstructive pulmonary disease
- PMID: 32997525
- PMCID: PMC7877965
- DOI: 10.1080/07853890.2020.1831050
Bacterial and viral infections and related inflammatory responses in chronic obstructive pulmonary disease
Abstract
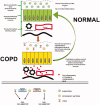
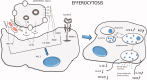
In chronic obstructive pulmonary disease (COPD) patients, bacterial and viral infections play a relevant role in worsening lung function and, therefore, favour disease progression. The inflammatory response to lung infections may become a specific indication of the bacterial and viral infections. We here review data on the bacterial-viral infections and related airways and lung parenchyma inflammation in stable and exacerbated COPD, focussing our attention on the prevalent molecular pathways in these different clinical conditions. The roles of macrophages, autophagy and NETosis are also briefly discussed in the context of lung infections in COPD. Controlling their combined response may restore a balanced lung homeostasis, reducing the risk of lung function decline. KEY MESSAGE Bacteria and viruses can influence the responses of the innate and adaptive immune system in the lung of chronic obstructive pulmonary disease (COPD) patients. The relationship between viruses and bacterial colonization, and the consequences of the imbalance of these components can modulate the inflammatory state of the COPD lung. The complex actions involving immune trigger cells, which activate innate and cell-mediated inflammatory responses, could be responsible for the clinical consequences of irreversible airflow limitation, lung remodelling and emphysema in COPD patients.
Keywords: B cells; ILCs; NETosis; NK cells; T-lymphocytes; autoimmunity; autophagy; dendritic cells; disability; macrophages; outcome; pyroptosis.
Conflict of interest statement
Gaetano Caramori reports grants, personal fees and non-financial support from Astra Zeneca, Boehringer Ingelheim, GSK and Menarini Group, and grants from Alfa-Sigma, outside the submitted work. The authors report no other conflicts of interest in this work.
Figures

References
-
- Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138:16–27. - PubMed
-
- Di Stefano A, Caramori G, Ricciardolo FL, et al. Cellular and molecular mechanisms in chronic obstructive pulmonary disease: an overview. Clin Exp Allergy. 2004;34:1156–1167. - PubMed
-
- Agusti A, Faner R, Celli B, et al. Precision medicine in COPD exacerbations. Lancet Respir Med. 2018;6:657–659. - PubMed
-
- Seemungal TA, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–1422. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
