Five-Year Outcomes With Nivolumab in Patients With Wild-Type BRAF Advanced Melanoma
- PMID: 32997575
- PMCID: PMC7676881
- DOI: 10.1200/JCO.20.00995
Five-Year Outcomes With Nivolumab in Patients With Wild-Type BRAF Advanced Melanoma
Abstract
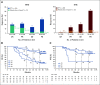
Purpose: The CheckMate 066 trial investigated nivolumab monotherapy as first-line treatment for patients with previously untreated BRAF wild-type advanced melanoma. Five-year results are presented herein.
Patients and methods: In this multicenter, double-blind, phase III study, 418 patients with previously untreated, unresectable, stage III/IV, wild-type BRAF melanoma were randomly assigned 1:1 to receive nivolumab 3 mg/kg every 2 weeks or dacarbazine 1,000 mg/m2 every 3 weeks. The primary end point was overall survival (OS), and secondary end points included progression-free survival (PFS), objective response rate (ORR), and safety.
Results: Patients were followed for a minimum of 60 months from the last patient randomly assigned (median follow-up, 32.0 months for nivolumab and 10.9 months for dacarbazine). Five-year OS rates were 39% with nivolumab and 17% with dacarbazine; PFS rates were 28% and 3%, respectively. Five-year OS was 38% in patients randomly assigned to dacarbazine who had subsequent therapy, including nivolumab (n = 37). ORR was 42% with nivolumab and 14% with dacarbazine; among patients alive at 5 years, ORR was 81% and 39%, respectively. Of 42 patients treated with nivolumab who had a complete response (20%), 88% (37 of 42) were alive as of the 5-year analysis. Among 75 nivolumab-treated patients alive and evaluable at the 5-year analysis, 83% had not received subsequent therapy; 23% were still on study treatment, and 60% were treatment free. Safety analyses were similar to the 3-year report.
Conclusion: Results from this 5-year analysis confirm the significant benefit of nivolumab over dacarbazine for all end points and add to the growing body of evidence supporting long-term survival with nivolumab mono-therapy. Survival is strongly associated with achieving a durable response, which can be maintained after treatment discontinuation, even without subsequent systemic therapies.
Trial registration: ClinicalTrials.gov NCT01721772.
Figures



References
-
- Larkin J Chiarion-Sileni V Gonzalez R, et al. : Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381:1535-1546, 2019 - PubMed
-
- Robert C Ribas A Schachter J, et al. : Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 20:1239-1251, 2019 - PubMed
-
- Robert C Schachter J Long GV, et al. : Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372:2521-2532, 2015 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

