Senolytic Drugs: Reducing Senescent Cell Viability to Extend Health Span
- PMID: 32997601
- PMCID: PMC7790861
- DOI: 10.1146/annurev-pharmtox-050120-105018
Senolytic Drugs: Reducing Senescent Cell Viability to Extend Health Span
Abstract
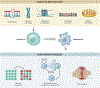
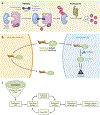
Senescence is the consequence of a signaling mechanism activated in stressed cells to prevent proliferation of cells with damage. Senescent cells (Sncs) often develop a senescence-associated secretory phenotype to prompt immune clearance, which drives chronic sterile inflammation and plays a causal role in aging and age-related diseases. Sncs accumulate with age and at anatomical sites of disease. Thus, they are regarded as a logical therapeutic target. Senotherapeutics are a new class of drugs that selectively kill Sncs (senolytics) or suppress their disease-causing phenotypes (senomorphics/senostatics). Since 2015, several senolytics went from identification to clinical trial. Preclinical data indicate that senolytics alleviate disease in numerous organs, improve physical function and resilience, and suppress all causes of mortality, even if administered to the aged. Here, we review the evidence that Sncs drive aging and disease, the approaches to identify and optimize senotherapeutics, and the current status of preclinical and clinical testing of senolytics.
Keywords: aging; senescence; senescence-associated secretory phenotype; senolytics; senomorphics.
Conflict of interest statement
DISCLOSURE STATEMENT
J.L.K. and T.T. have a financial interest related to this research. Patents on senolytic drugs are held by the Mayo Clinic and the University of Minnesota. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies.
Figures


References
-
- Olshansky SJ. 2013. Life expectancy and education: the author replies. Health Aff. 32:822 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

