Ultrasound-Guided Breast Biopsies: Basic and New Techniques
- PMID: 32997819
- PMCID: PMC8246574
- DOI: 10.1002/jum.15517
Ultrasound-Guided Breast Biopsies: Basic and New Techniques
Abstract
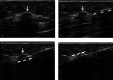
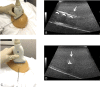
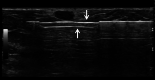
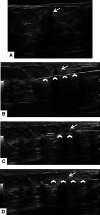
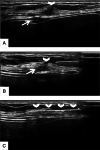
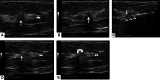
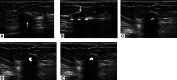
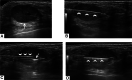
Ultrasound-guided breast biopsies can be challenging to perform, especially when the target is adjacent to the nipple, skin, or implant or when the target is small and in very posterior, dense fibroglandular tissue. Oftentimes, a slightly modified approach can result in a diagnostic biopsy specimen with minimal complications. After a brief review of basic techniques for ultrasound-guided breast biopsies that includes a review of conventional breast biopsy devices, a presentation of procedural modifications and techniques to consider for more challenging cases is described. In particular, novel open-trough and tandem-needle techniques are detailed. Several cases using these techniques are then presented.
Keywords: breast biopsy; breast intervention; hydrodissection; tandem needle; ultrasound-guided.
© 2020 Mayo Clinic. Journal of Ultrasound in Medicine published by Wiley Periodicals LLC on behalf of American Institute of Ultrasound in Medicine..
Figures






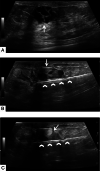
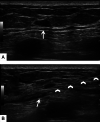
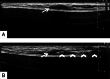
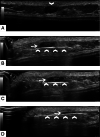


References
-
- Parker SH, Jobe WE, Dennis MA, et al. US‐guided automated large‐core breast biopsy. Radiology 1993; 187:507–511. - PubMed
-
- Parker SH, Burbank F, Jackman RJ, et al. Percutaneous large‐core breast biopsy: a multi‐institutional study. Radiology 1994; 193:359–364. - PubMed
-
- Caruncho MV, Branas F. Mirror pain as a complication of large core biopsy of the breast. Breast J 2006; 12:393–395. - PubMed
-
- Harvey JA, Moran RE. US‐guided core needle biopsy of the breast: technique and pitfalls. Radiographics 1998; 18:867–877. - PubMed
-
- Brenner RJ, Fajardo L, Fisher PR, et al. Percutaneous core biopsy of the breast: effect of operator experience and number of samples on diagnostic accuracy. AJR Am J Roentgenol 1996; 166:341–346. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

