Amino Acids and Their Transporters in T Cell Immunity and Cancer Therapy
- PMID: 32997964
- PMCID: PMC7655528
- DOI: 10.1016/j.molcel.2020.09.006
Amino Acids and Their Transporters in T Cell Immunity and Cancer Therapy
Abstract
Metabolism reprogramming is critical for both cancer progression and effective immune responses in the tumor microenvironment. Amino acid metabolism in different cells and their cross-talk shape tumor immunity and therapy efficacy in patients with cancer. In this review, we focus on multiple amino acids and their transporters, solute carrier (SLC) members. We discuss their involvement in regulation of immune responses in the tumor microenvironment and assess their associations with cancer immunotherapy, chemotherapy, and radiation therapy, and we review their potential as targets for cancer therapy. We stress the necessity to understand individual amino acids and their transporters in different cell subsets, the molecular intersection between amino acid metabolism, and effective T cell immunity and its relevance in cancer therapies.
Keywords: CD8(+) T cell; amino acid; cancer; checkpoint blockade; immunotherapy; metabolism; solute carriers.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures


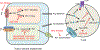

References
-
- Abou-Alfa GK, Qin S, Ryoo BY, Lu SN, Yen CJ, Feng YH, Lim HY, Izzo F, Colombo M, Sarker D, et al. (2018). Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 29, 1402–1408. - PubMed
-
- Arensman MD, Yang XS, Leahy DM, Toral-Barza L, Mileski M, Rosfjord EC, Wang F, Deng S, Myers JS, Abraham RT, et al. (2019). Cystine-glutamate antiporter xCT deficiency suppresses tumor growth while preserving antitumor immunity. Proceedings of the National Academy of Sciences of the United States of America 116, 9533–9542. - PMC - PubMed
-
- Berenbaum MC, Ginsburg H, and Gilbert DM (1970). Effects of L-asparaginase on lymphocyte-target cell reactions in vitro. Nature 227, 1147–1148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

