Neuroprotective Effect of Antioxidants in the Brain
- PMID: 32998277
- PMCID: PMC7582347
- DOI: 10.3390/ijms21197152
Neuroprotective Effect of Antioxidants in the Brain
Abstract
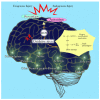
The brain is vulnerable to excessive oxidative insults because of its abundant lipid content, high energy requirements, and weak antioxidant capacity. Reactive oxygen species (ROS) increase susceptibility to neuronal damage and functional deficits, via oxidative changes in the brain in neurodegenerative diseases. Overabundance and abnormal levels of ROS and/or overload of metals are regulated by cellular defense mechanisms, intracellular signaling, and physiological functions of antioxidants in the brain. Single and/or complex antioxidant compounds targeting oxidative stress, redox metals, and neuronal cell death have been evaluated in multiple preclinical and clinical trials as a complementary therapeutic strategy for combating oxidative stress associated with neurodegenerative diseases. Herein, we present a general analysis and overview of various antioxidants and suggest potential courses of antioxidant treatments for the neuroprotection of the brain from oxidative injury. This review focuses on enzymatic and non-enzymatic antioxidant mechanisms in the brain and examines the relative advantages and methodological concerns when assessing antioxidant compounds for the treatment of neurodegenerative disorders.
Keywords: antioxidant; brain; neurodegenerative disease; neuroprotection; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Dual effects of antioxidants in neurodegeneration: direct neuroprotection against oxidative stress and indirect protection via suppression of glia-mediated inflammation.Curr Pharm Des. 2006;12(27):3521-33. doi: 10.2174/138161206778343109. Curr Pharm Des. 2006. PMID: 17017945 Review.
-
Potential for therapeutic use of hydrogen sulfide in oxidative stress-induced neurodegenerative diseases.Int J Med Sci. 2019 Sep 20;16(10):1386-1396. doi: 10.7150/ijms.36516. eCollection 2019. Int J Med Sci. 2019. PMID: 31692944 Free PMC article. Review.
-
Autophagy as an essential cellular antioxidant pathway in neurodegenerative disease.Redox Biol. 2013 Dec 25;2:82-90. doi: 10.1016/j.redox.2013.12.013. eCollection 2014. Redox Biol. 2013. PMID: 24494187 Free PMC article. Review.
-
Natural Antioxidant Anthocyanins-A Hidden Therapeutic Candidate in Metabolic Disorders with Major Focus in Neurodegeneration.Nutrients. 2019 May 28;11(6):1195. doi: 10.3390/nu11061195. Nutrients. 2019. PMID: 31141884 Free PMC article. Review.
-
Safeguarding the brain from oxidative damage.Free Radic Biol Med. 2025 Jan;226:143-157. doi: 10.1016/j.freeradbiomed.2024.11.019. Epub 2024 Nov 13. Free Radic Biol Med. 2025. PMID: 39547523 Review.
Cited by
-
The neuroprotective potential of turmeric rhizome and bitter melon on aspartame-induced spatial memory impairment in rats.Heliyon. 2023 Nov 3;9(11):e21693. doi: 10.1016/j.heliyon.2023.e21693. eCollection 2023 Nov. Heliyon. 2023. PMID: 38027700 Free PMC article.
-
Effect of resistance and endurance training with ursolic acid on oxidative stress and cognitive impairment in hippocampal tissue in HFD/STZ-induced aged diabetic rats.Iran J Basic Med Sci. 2023;26(12):1449-1459. doi: 10.22038/IJBMS.2023.71230.15477. Iran J Basic Med Sci. 2023. PMID: 37970434 Free PMC article.
-
The role of the SIRT1-BMAL1 pathway in regulating oxidative stress in the early development of ischaemic stroke.Sci Rep. 2024 Jan 20;14(1):1773. doi: 10.1038/s41598-024-52120-5. Sci Rep. 2024. PMID: 38245621 Free PMC article.
-
Oxidative stress, the blood-brain barrier and neurodegenerative diseases: The critical beneficial role of dietary antioxidants.Acta Pharm Sin B. 2023 Oct;13(10):3988-4024. doi: 10.1016/j.apsb.2023.07.010. Epub 2023 Jul 16. Acta Pharm Sin B. 2023. PMID: 37799389 Free PMC article. Review.
-
Neurotoxic Effects of Neonicotinoids on Mammals: What Is There beyond the Activation of Nicotinic Acetylcholine Receptors?-A Systematic Review.Int J Mol Sci. 2021 Aug 5;22(16):8413. doi: 10.3390/ijms22168413. Int J Mol Sci. 2021. PMID: 34445117 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

