Limited Availability of General Co-Repressors Uncovered in an Overexpression Context during Wing Venation in Drosophila melanogaster
- PMID: 32998295
- PMCID: PMC7601384
- DOI: 10.3390/genes11101141
Limited Availability of General Co-Repressors Uncovered in an Overexpression Context during Wing Venation in Drosophila melanogaster
Abstract
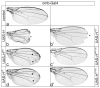
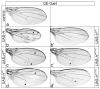
Cell fate is determined by the coordinated activity of different pathways, including the conserved Notch pathway. Activation of Notch results in the transcription of Notch targets that are otherwise silenced by repressor complexes. In Drosophila, the repressor complex comprises the transcription factor Suppressor of Hairless (Su(H)) bound to the Notch antagonist Hairless (H) and the general co-repressors Groucho (Gro) and C-terminal binding protein (CtBP). The latter two are shared by different repressors from numerous pathways, raising the possibility that they are rate-limiting. We noted that the overexpression during wing development of H mutants HdNT and HLD compromised in Su(H)-binding induced ectopic veins. On the basis of the role of H as Notch antagonist, overexpression of Su(H)-binding defective H isoforms should be without consequence, implying different mechanisms but repression of Notch signaling activity. Perhaps excess H protein curbs general co-repressor availability. Supporting this model, nearly normal wings developed upon overexpression of H mutant isoforms that bound neither Su(H) nor co-repressor Gro and CtBP. Excessive H protein appeared to sequester general co-repressors, resulting in specific vein defects, indicating their limited availability during wing vein development. In conclusion, interpretation of overexpression phenotypes requires careful consideration of possible dominant negative effects from interception of limiting factors.
Keywords: C-terminal binding protein; Drosophila; Groucho; Hairless; Notch signaling; Suppressor of Hairless; co-repressor; repressor complex; sequestration; wing venation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




Similar articles
-
Fine tuning of Notch signaling by differential co-repressor recruitment during eye development of Drosophila.Hereditas. 2011 Jun;148(3):77-84. doi: 10.1111/j.1601-5223.2011.02221.x. Epub 2011 May 26. Hereditas. 2011. PMID: 21756252
-
The Notch repressor complex in Drosophila: in vivo analysis of Hairless mutants using overexpression experiments.Dev Genes Evol. 2019 Jan;229(1):13-24. doi: 10.1007/s00427-018-00624-2. Epub 2019 Jan 5. Dev Genes Evol. 2019. PMID: 30612166
-
Regulation of expression of Vg and establishment of the dorsoventral compartment boundary in the wing imaginal disc by Suppressor of Hairless.Dev Biol. 2006 Jan 1;289(1):77-90. doi: 10.1016/j.ydbio.2005.10.008. Epub 2005 Nov 22. Dev Biol. 2006. PMID: 16307735
-
Deconstructing repression: evolving models of co-repressor action.Nat Rev Genet. 2010 Feb;11(2):109-23. doi: 10.1038/nrg2736. Nat Rev Genet. 2010. PMID: 20084085 Review.
-
Notch and Senescence.Adv Exp Med Biol. 2018;1066:299-318. doi: 10.1007/978-3-319-89512-3_15. Adv Exp Med Biol. 2018. PMID: 30030833 Review.
Cited by
-
Novel Genome-Engineered H Alleles Differentially Affect Lateral Inhibition and Cell Dichotomy Processes during Bristle Organ Development.Genes (Basel). 2024 Apr 26;15(5):552. doi: 10.3390/genes15050552. Genes (Basel). 2024. PMID: 38790181 Free PMC article.
-
Phospho-Site Mutations in Transcription Factor Suppressor of Hairless Impact Notch Signaling Activity During Hematopoiesis in Drosophila.Front Cell Dev Biol. 2021 Apr 14;9:658820. doi: 10.3389/fcell.2021.658820. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33937259 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

