Extracts from Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. Roots: A New Hope Against Honeybee Death Caused by Nosemosis
- PMID: 32998304
- PMCID: PMC7582972
- DOI: 10.3390/molecules25194452
Extracts from Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. Roots: A New Hope Against Honeybee Death Caused by Nosemosis
Abstract
Pollinators, the cornerstones of our terrestrial ecosystem, have been at the very core of our anxiety. This is because we can nowadays observe a dangerous decline in the number of insects. With the numbers of pollinators dramatically declining worldwide, the scientific community has been growing more and more concerned about the future of insects as fundamental elements of most terrestrial ecosystems. Trying to address this issue, we looked for substances that might increase bee resistance. To this end, we checked the effects of plant-based adaptogens on honeybees in laboratory tests and during field studies on 30 honeybee colonies during two seasons. In this study, we have tested extracts obtained from: Eleutherococcus senticosus, Garcinia cambogia, Panax ginseng, Ginkgo biloba, Schisandra chinensis, and Camellia sinensis. The 75% ethanol E. senticosus root extract proved to be the most effective, both as a cure and in the prophylaxis of nosemosis. Therefore, Eleutherococcus senticosus, and its active compounds, eleutherosides, are considered the most powerful adaptogens, in the pool of all extracts that were selected for screening, for supporting immunity and improving resistance of honeybees. The optimum effective concentration of 0.4 mg/mL E. senticosus extract responded to c.a. 5.76, 2.56 and 0.07 µg/mL of eleutheroside B, eleutheroside E and naringenin, respectively. The effect of E. senticosus extracts on honeybees involved a similar adaptogenic response as on other animals, including humans. In this research, we show for the first time such an adaptogenic impact on invertebrates, i.e., the effect on honeybees stressed by nosemosis. We additionally hypothesised that these adaptogenic properties were connected with eleutherosides-secondary metabolites found exclusively in the Eleutherococcus genus and undetected in other studied extracts. As was indicated in this study, eleutherosides are very stable chemically and can be found in extracts in similar amounts even after two years from extraction. Considering the role bees play in nature, we may conclude that demonstrating the adaptogenic properties which plant extracts have in insects is the most significant finding resulting from this research. This knowledge might bring to fruition numerous economic and ecological benefits.
Keywords: Apis mellifera; Camellia sinensis; Eleutherococcus senticosus; Garcinia cambogia; Ginkgo biloba; Panax ginseng; Schisandra chinensis; adaptogenic herbs; eleutherosides; fumagillin; honeybee; insectageddon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



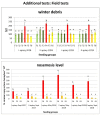
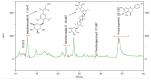

Similar articles
-
A comparative study on root and bark extracts of Eleutherococcus senticosus and their effects on human macrophages.Phytomedicine. 2020 Mar;68:153181. doi: 10.1016/j.phymed.2020.153181. Epub 2020 Feb 6. Phytomedicine. 2020. PMID: 32065954
-
The intractum from the Eleutherococcus senticosus fruits affects the innate immunity in human leukocytes: From the ethnomedicinal use to contemporary evidence-based research.J Ethnopharmacol. 2021 Mar 25;268:113636. doi: 10.1016/j.jep.2020.113636. Epub 2020 Dec 1. J Ethnopharmacol. 2021. PMID: 33271247
-
Evaluation of molecular chaperons Hsp72 and neuropeptide Y as characteristic markers of adaptogenic activity of plant extracts.Phytomedicine. 2013 Nov 15;20(14):1323-9. doi: 10.1016/j.phymed.2013.07.001. Epub 2013 Aug 6. Phytomedicine. 2013. PMID: 23920279
-
Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. (Araliaceae) as an adaptogen: a closer look.J Ethnopharmacol. 2000 Oct;72(3):345-93. doi: 10.1016/s0378-8741(00)00181-1. J Ethnopharmacol. 2000. PMID: 10996277 Review.
-
Eleutherococcus senticosus (Acanthopanax senticosus): An Important Adaptogenic Plant.Molecules. 2025 Jun 8;30(12):2512. doi: 10.3390/molecules30122512. Molecules. 2025. PMID: 40572479 Free PMC article. Review.
Cited by
-
Plants and Their Derivatives as Promising Therapeutics for Sustainable Control of Honeybee (Apis mellifera) Pathogens.Pathogens. 2023 Oct 19;12(10):1260. doi: 10.3390/pathogens12101260. Pathogens. 2023. PMID: 37887776 Free PMC article. Review.
-
Amplicon Sequencing of Variable 16S rRNA from Bacteria and ITS2 Regions from Fungi and Plants, Reveals Honeybee Susceptibility to Diseases Results from Their Forage Availability under Anthropogenic Landscapes.Pathogens. 2021 Mar 22;10(3):381. doi: 10.3390/pathogens10030381. Pathogens. 2021. PMID: 33810160 Free PMC article.
-
The Herbal Supplements NOZEMAT HERB® and NOZEMAT HERB PLUS®: An Alternative Therapy for N. ceranae Infection and Its Effects on Honey Bee Strength and Production Traits.Pathogens. 2021 Feb 19;10(2):234. doi: 10.3390/pathogens10020234. Pathogens. 2021. PMID: 33669663 Free PMC article.
-
Biological control of nosemosis in Apis mellifera L. with Acacia nilotica extract.Sci Rep. 2024 Nov 16;14(1):28340. doi: 10.1038/s41598-024-78874-6. Sci Rep. 2024. PMID: 39550385 Free PMC article.
-
Use of Thymol in Nosema ceranae Control and Health Improvement of Infected Honey Bees.Insects. 2022 Jun 24;13(7):574. doi: 10.3390/insects13070574. Insects. 2022. PMID: 35886750 Free PMC article.
References
-
- Aslan C.E., Liang C.T., Galindo B., Kimberly H., Topete W. The role of honey bees as pollinators in natural areas. Nat. Areas J. 2016;36:478–488. doi: 10.3375/043.036.0413. - DOI
-
- Prasad P.Y., Mackereth R.W., Hanley R.S., Qin W. Honey Bees (Apis mellifera L.) and Pollination Issues: Current status, impacts and potential drivers of decline. J. Agric. Sci. 2015;7:93.
-
- Zalasiewicz J., Waters C.N., Summerhayes C.P., Wolfe A.P., Barnosky A.D., Cearreta A., Crutzen P., Ellis E., Fairchild I.J., Gałuszka A., et al. The Working Group on the Anthropocene: Summary of evidence and interim recommendations. Anthropocene. 2017;19:55–60. doi: 10.1016/j.ancene.2017.09.001. - DOI
-
- Crutzen P.J., Stoermer E.F. The Anthropocene. Global Change Newsletter. Int. Geosph. Biosph. Programme. 2000;41:17–18.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

