Caffeoyl-Prolyl-Histidine Amide Inhibits Fyn and Alleviates Atopic Dermatitis-Like Phenotypes via Suppression of NF-κB Activation
- PMID: 32998341
- PMCID: PMC7582254
- DOI: 10.3390/ijms21197160
Caffeoyl-Prolyl-Histidine Amide Inhibits Fyn and Alleviates Atopic Dermatitis-Like Phenotypes via Suppression of NF-κB Activation
Abstract
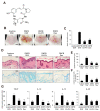
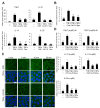
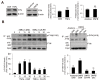
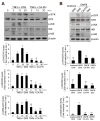
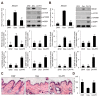
Caffeic acid (CA) is produced from a variety of plants and has diverse biological functions, including anti-inflammation activity. It has been recently demonstrated that caffeoyl-prolyl-histidine amide (CA-PH), which is CA conjugated with proline-histidine dipeptide, relieves atopic dermatitis (AD)-like phenotypes in mouse. In this study, we investigated the molecular mechanism underlying CA-PH-mediated alleviation of AD-like phenotypes using cell line and AD mouse models. We confirmed that CA-PH suppresses AD-like phenotypes, such as increased epidermal thickening, infiltration of mast cells, and dysregulated gene expression of cytokines. CA-PH suppressed up-regulation of cytokine expression through inhibition of nuclear translocation of NF-κB. Using a CA-PH affinity pull-down assay, we found that CA-PH binds to Fyn. In silico molecular docking and enzyme kinetic studies revealed that CA-PH binds to the ATP binding site and inhibits Fyn competitively with ATP. CA-PH further suppressed spleen tyrosine kinase (SYK)/inhibitor of nuclear factor kappa B kinase (IKK)/inhibitor of nuclear factor kappa B (IκB) signaling, which is required for nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation. In addition, chronic application of CA-PH, in contrast with that of glucocorticoids, did not induce up-regulation of regulated in development and DNA damage response 1 (REDD1), reduction of mammalian target of rapamycin (mTOR) signaling, or skin atrophy. Thus, our study suggests that CA-PH treatment may help to reduce skin inflammation via down-regulation of NF-κB activation, and Fyn may be a new therapeutic target of inflammatory skin diseases, such as AD.
Keywords: CA-PH; Fyn; NF-κB; SYK; atopic dermatitis; skin atrophy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
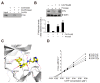
Similar articles
-
Chlorogenic acid alleviates DNCB-induced atopic dermatitis by inhibiting the Akt1/NF-κB signaling pathway.Eur J Pharmacol. 2025 Jul 5;998:177534. doi: 10.1016/j.ejphar.2025.177534. Epub 2025 Mar 19. Eur J Pharmacol. 2025. PMID: 40118327
-
Conjugated linoleic acid attenuates 2,4-dinitrofluorobenzene-induced atopic dermatitis in mice through dual inhibition of COX-2/5-LOX and TLR4/NF-κB signaling.J Nutr Biochem. 2020 Jul;81:108379. doi: 10.1016/j.jnutbio.2020.108379. Epub 2020 Mar 24. J Nutr Biochem. 2020. PMID: 32330842
-
Anti-inflammatory effect of Centella asiatica phytosome in a mouse model of phthalic anhydride-induced atopic dermatitis.Phytomedicine. 2018 Apr 1;43:110-119. doi: 10.1016/j.phymed.2018.04.013. Epub 2018 Apr 6. Phytomedicine. 2018. PMID: 29747743
-
Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity.Annu Rev Immunol. 2000;18:621-63. doi: 10.1146/annurev.immunol.18.1.621. Annu Rev Immunol. 2000. PMID: 10837071 Review.
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
Cited by
-
Ghrelin Represses Thymic Stromal Lymphopoietin Gene Expression through Activation of Glucocorticoid Receptor and Protein Kinase C Delta in Inflamed Skin Keratinocytes.Int J Mol Sci. 2022 Apr 2;23(7):3977. doi: 10.3390/ijms23073977. Int J Mol Sci. 2022. PMID: 35409338 Free PMC article.
-
The immunomodulatory activity of Orthosiphon aristatus against atopic dermatitis: Evidence-based on network pharmacology and molecular simulations.J Taibah Univ Med Sci. 2023 Nov 4;19(1):164-174. doi: 10.1016/j.jtumed.2023.10.005. eCollection 2024 Feb. J Taibah Univ Med Sci. 2023. PMID: 38047238 Free PMC article.
-
Identifying the hub genes for Duchenne muscular dystrophy and Becker muscular dystrophy by weighted correlation network analysis.BMC Genom Data. 2021 Dec 18;22(1):57. doi: 10.1186/s12863-021-01014-w. BMC Genom Data. 2021. PMID: 34922439 Free PMC article.
-
Investigating the efficacy of gliclazide encapsulated hydrogel in the preclinical mice model for atopic dermatitis.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jun;398(6):7489-7504. doi: 10.1007/s00210-024-03741-0. Epub 2025 Jan 4. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39754682
-
Tau Protein as Therapeutic Target for Cancer? Focus on Glioblastoma.Cancers (Basel). 2022 Nov 1;14(21):5386. doi: 10.3390/cancers14215386. Cancers (Basel). 2022. PMID: 36358803 Free PMC article. Review.
References
-
- Yamada Y., Yasui H., Sakurai H. Suppressive Effect of Caffeic Acid and its Derivatives on the Generation of UVA-induced Reactive Oxygen Species in the Skin of Hairless Mice and Pharmacokinetic Analysis on Organ Distribution of Caffeic Acid in ddY Mice. Photochem. Photobiol. 2006;82:1668. doi: 10.1111/j.1751-1097.2006.tb09829.x. - DOI - PubMed
-
- Lima V.N., Oliveira-Tintino C.D., Santos E.S., Morais L.P., Tintino S.R., Freitas T.S., Geraldo Y.S., Pereira R.L., Cruz R.P., Menezes I.R., et al. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microb. Pathog. 2016;99:56–61. doi: 10.1016/j.micpath.2016.08.004. - DOI - PubMed
-
- Hirose M., Takesada Y., Tanaka H., Tamano S., Kato T., Shirai T. Carcinogenicity of antioxidants BHA, caffeic acid, sesamol, 4-methoxyphenol and catechol at low doses, either alone or in combination, and modulation of their effects in a rat medium-term multi-organ carcinogenesis model. Carcinogenesis. 1998;19:207–212. doi: 10.1093/carcin/19.1.207. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

