Molecular Ultrasound Imaging
- PMID: 32998422
- PMCID: PMC7601169
- DOI: 10.3390/nano10101935
Molecular Ultrasound Imaging
Abstract
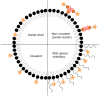
In the last decade, molecular ultrasound imaging has been rapidly progressing. It has proven promising to diagnose angiogenesis, inflammation, and thrombosis, and many intravascular targets, such as VEGFR2, integrins, and selectins, have been successfully visualized in vivo. Furthermore, pre-clinical studies demonstrated that molecular ultrasound increased sensitivity and specificity in disease detection, classification, and therapy response monitoring compared to current clinically applied ultrasound technologies. Several techniques were developed to detect target-bound microbubbles comprising sensitive particle acoustic quantification (SPAQ), destruction-replenishment analysis, and dwelling time assessment. Moreover, some groups tried to assess microbubble binding by a change in their echogenicity after target binding. These techniques can be complemented by radiation force ultrasound improving target binding by pushing microbubbles to vessel walls. Two targeted microbubble formulations are already in clinical trials for tumor detection and liver lesion characterization, and further clinical scale targeted microbubbles are prepared for clinical translation. The recent enormous progress in the field of molecular ultrasound imaging is summarized in this review article by introducing the most relevant detection technologies, concepts for targeted nano- and micro-bubbles, as well as their applications to characterize various diseases. Finally, progress in clinical translation is highlighted, and roadblocks are discussed that currently slow the clinical translation.
Keywords: active targeting; angiogenesis; clinical translation; inflammation; molecular imaging; molecular ultrasound; nanobubbles; targeted microbubbles; thrombosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Targeted ultrasound contrast agent for molecular imaging of inflammation in high-shear flow.Contrast Media Mol Imaging. 2006 Nov-Dec;1(6):259-66. doi: 10.1002/cmmi.113. Contrast Media Mol Imaging. 2006. PMID: 17191766
-
Ultrasound microbubbles for molecular diagnosis, therapy, and theranostics.J Nucl Med. 2012 Mar;53(3):345-8. doi: 10.2967/jnumed.111.099754. J Nucl Med. 2012. PMID: 22393225 Review.
-
Molecular Acoustic Angiography: A New Technique for High-resolution Superharmonic Ultrasound Molecular Imaging.Ultrasound Med Biol. 2016 Mar;42(3):769-81. doi: 10.1016/j.ultrasmedbio.2015.10.015. Epub 2015 Dec 8. Ultrasound Med Biol. 2016. PMID: 26678155 Free PMC article.
-
BR55: a lipopeptide-based VEGFR2-targeted ultrasound contrast agent for molecular imaging of angiogenesis.Invest Radiol. 2010 Feb;45(2):89-95. doi: 10.1097/RLI.0b013e3181c5927c. Invest Radiol. 2010. PMID: 20027118
-
Ultrasound molecular imaging.Methods. 2009 Jun;48(2):92-7. doi: 10.1016/j.ymeth.2009.03.011. Epub 2009 Mar 24. Methods. 2009. PMID: 19324089 Review.
Cited by
-
Advanced ultrasound methods to improve chronic kidney disease diagnosis.Npj Imaging. 2024 Jul 25;2(1):22. doi: 10.1038/s44303-024-00023-5. Npj Imaging. 2024. PMID: 40604097 Free PMC article. Review.
-
Regenerative Approaches and Future Trends for the Treatment of Corneal Burn Injuries.J Clin Med. 2021 Jan 16;10(2):317. doi: 10.3390/jcm10020317. J Clin Med. 2021. PMID: 33467167 Free PMC article. Review.
-
Transferrin Receptor-Targeted Nonspherical Microbubbles for Blood-Brain Barrier Sonopermeation.Adv Mater. 2023 Dec;35(52):e2308150. doi: 10.1002/adma.202308150. Epub 2023 Nov 27. Adv Mater. 2023. PMID: 37949438 Free PMC article.
-
Air-Filled Microbubbles Based on Albumin Functionalized with Gold Nanocages and Zinc Phthalocyanine for Multimodal Imaging.Micromachines (Basel). 2021 Sep 27;12(10):1161. doi: 10.3390/mi12101161. Micromachines (Basel). 2021. PMID: 34683212 Free PMC article.
-
The Current Status and Future Directions on Nanoparticles for Tumor Molecular Imaging.Int J Nanomedicine. 2024 Sep 14;19:9549-9574. doi: 10.2147/IJN.S484206. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39296941 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

