Thymoquinone-Loaded Polymeric Films and Hydrogels for Bacterial Disinfection and Wound Healing
- PMID: 32998437
- PMCID: PMC7600314
- DOI: 10.3390/biomedicines8100386
Thymoquinone-Loaded Polymeric Films and Hydrogels for Bacterial Disinfection and Wound Healing
Abstract
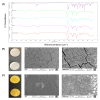
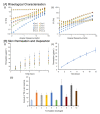
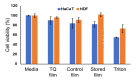
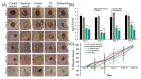
The purpose of this study was to synthesize and characterize novel biocompatible topical polymeric film and hydrogel systems that have the potential to deliver the antibacterial agent thymoquinone (TQ) directly to the skin target site to manage the local wound infection and thereby wound healing. The polyvinyl pyrrolidone (PVP) matrix-type films containing TQ were prepared by the solvent casting method. In vitro skin permeation studies on human cadaver skin produced a mean flux of 2.3 µg TQ/cm2/h. Human keratinocyte monolayers subjected to a scratch wound (an in vitro wound healing assay) showed 85% wound closure at day 6 in the TQ group (100 ng/mL TQ) as compared to 50% in the vehicle control group (p = 0.0001). In a zone-of-inhibition (ZOI) assay, TQ-containing films and hydrogels completely wiped out Staphylococcus aureus in 10 cm diameter Tryptic Soy Agar plates while 500 µg/mL gentamicin containing filters gave 10 mm of ZOI. In an ex vivo model, TQ-containing films eradicated bacterial colonization on human cadaver skin. Furthermore, in a full-thickness wound infection model in mice, TQ-containing films showed significant activity in controlling Staphylococcus aureus infection, thereby disinfecting the skin wound. In summary, TQ-containing PVP films and hydrogels developed in this study have the potential to treat and manage wound infections.
Keywords: Staphylococcus aureus; bacterial skin infections; polymeric film and hydrogel; thymoquinone; topical/transdermal drug delivery; wound disinfection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Kumar S., Kang H.J., Berthiaume F. 40 - Scaffolds for epidermal tissue engineering. In: Mozafari M., Sefat F., Atala A., editors. Handbook of Tissue Engineering Scaffolds: Volume Two. Woodhead Publishing; Cambridge, UK: 2019. [(accessed on 27 September 2020)]. pp. 173–191. Available online: - DOI
-
- Naimi T.S., LeDell K.H., Como-Sabetti K., Borchardt S.M., Boxrud D.J., Etienne J., Johnson S.K., Vandenesch F., Fridkin S., O’Boyle C., et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290:2976–2984. doi: 10.1001/jama.290.22.2976. - DOI - PubMed
LinkOut - more resources
Full Text Sources

