Oxidative Stress and Microglial Response in Retinitis Pigmentosa
- PMID: 32998461
- PMCID: PMC7583782
- DOI: 10.3390/ijms21197170
Oxidative Stress and Microglial Response in Retinitis Pigmentosa
Abstract
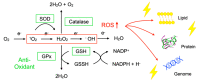
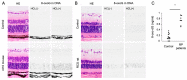
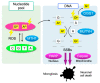
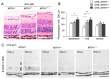
An imbalance between the production of reactive oxygen species (ROS) and anti-oxidant capacity results in oxidative injury to cellular components and molecules, which in turn disturbs the homeostasis of cells and organs. Although retinitis pigmentosa (RP) is a hereditary disease, non-genetic biological factors including oxidative stress also modulate or contribute to the disease progression. In animal models of RP, the degenerating retina exhibits marked oxidative damage in the nucleic acids, proteins, and lipids, and anti-oxidant treatments substantially suppress photoreceptor cell death and microgliosis. Although the mechanisms by which oxidative stress mediates retinal degeneration have not been fully elucidated, our group has shown that oxidative DNA damage and its defense system are key regulators of microglial activation and photoreceptor degeneration in RP. In this review, we summarize the current evidence regarding oxidative stress in animal models and patients with RP. The clinical efficacy of anti-oxidant treatments for RP has not been fully established. Nevertheless, elucidating key biological processes that underlie oxidative damage in RP will be pivotal to understanding the pathology and developing a potent anti-oxidant strategy that targets specific cell types or molecules under oxidative stress.
Keywords: microglia; oxidative DNA damage; oxidative stress; retinitis pigmentosa.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Minocycline prevents photoreceptor degeneration in Retinitis pigmentosa through modulating mitochondrial homeostasis.Int Immunopharmacol. 2024 Sep 30;139:112703. doi: 10.1016/j.intimp.2024.112703. Epub 2024 Jul 17. Int Immunopharmacol. 2024. PMID: 39018687
-
Gypenosides attenuate retinal degeneration in a zebrafish retinitis pigmentosa model.Exp Eye Res. 2020 Dec;201:108291. doi: 10.1016/j.exer.2020.108291. Epub 2020 Oct 10. Exp Eye Res. 2020. PMID: 33049273
-
MutT homolog-1 attenuates oxidative DNA damage and delays photoreceptor cell death in inherited retinal degeneration.Am J Pathol. 2012 Oct;181(4):1378-86. doi: 10.1016/j.ajpath.2012.06.026. Epub 2012 Jul 27. Am J Pathol. 2012. PMID: 22841817
-
Redox Status in Retinitis Pigmentosa.Adv Exp Med Biol. 2023;1415:443-448. doi: 10.1007/978-3-031-27681-1_65. Adv Exp Med Biol. 2023. PMID: 37440070 Review.
-
Role of antioxidant enzymes and small molecular weight antioxidants in the pathogenesis of age-related macular degeneration (AMD).Biogerontology. 2013 Oct;14(5):461-82. doi: 10.1007/s10522-013-9463-2. Epub 2013 Sep 22. Biogerontology. 2013. PMID: 24057278 Free PMC article. Review.
Cited by
-
Time-Course Changes in Oxidative Stress and Inflammation in the Retinas of rds Mice: A Retinitis Pigmentosa Model.Antioxidants (Basel). 2022 Sep 29;11(10):1950. doi: 10.3390/antiox11101950. Antioxidants (Basel). 2022. PMID: 36290673 Free PMC article.
-
Microtubule modification defects underlie cilium degeneration in cell models of retinitis pigmentosa associated with pre-mRNA splicing factor mutations.Front Genet. 2022 Sep 13;13:1009430. doi: 10.3389/fgene.2022.1009430. eCollection 2022. Front Genet. 2022. PMID: 36176300 Free PMC article.
-
More than meets the eye: The role of microglia in healthy and diseased retina.Front Immunol. 2022 Nov 29;13:1006897. doi: 10.3389/fimmu.2022.1006897. eCollection 2022. Front Immunol. 2022. PMID: 36524119 Free PMC article. Review.
-
Nutraceutical Supplementation Ameliorates Visual Function, Retinal Degeneration, and Redox Status in rd10 Mice.Antioxidants (Basel). 2021 Jun 26;10(7):1033. doi: 10.3390/antiox10071033. Antioxidants (Basel). 2021. PMID: 34206804 Free PMC article.
-
Redox Regulation of Immunometabolism in Microglia Underpinning Diabetic Retinopathy.Antioxidants (Basel). 2024 Mar 29;13(4):423. doi: 10.3390/antiox13040423. Antioxidants (Basel). 2024. PMID: 38671871 Free PMC article. Review.
References
-
- Takeya R., Sumimoto H. Molecular mechanism for activation of superoxide-producing NADPH oxidases. Mol. Cells. 2003;16:271–277. - PubMed
-
- Morizane Y., Morimoto N., Fujiwara A., Kawasaki R., Yamashita H., Ogura Y., Shiraga F. Incidence and causes of visual impairment in Japan: The first nation-wide complete enumeration survey of newly certified visually impaired individuals. Jpn. J. Ophthalmol. 2019;63:26–33. doi: 10.1007/s10384-018-0623-4. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

