Membrane Interaction of Ibuprofen with Cholesterol-Containing Lipid Membranes
- PMID: 32998467
- PMCID: PMC7650631
- DOI: 10.3390/biom10101384
Membrane Interaction of Ibuprofen with Cholesterol-Containing Lipid Membranes
Abstract
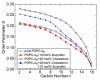
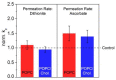
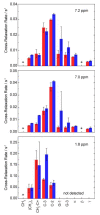
Deciphering the membrane interaction of drug molecules is important for improving drug delivery, cellular uptake, and the understanding of side effects of a given drug molecule. For the anti-inflammatory drug ibuprofen, several studies reported contradictory results regarding the impact of ibuprofen on cholesterol-containing lipid membranes. Here, we investigated membrane localization and orientation as well as the influence of ibuprofen on membrane properties in POPC/cholesterol bilayers using solid-state NMR spectroscopy and other biophysical assays. The presence of ibuprofen disturbs the molecular order of phospholipids as shown by alterations of the 2H and 31P-NMR spectra of the lipids, but does not lead to an increased membrane permeability or changes of the phase state of the bilayer. 1H MAS NOESY NMR results demonstrate that ibuprofen adopts a mean position in the upper chain/glycerol region of the POPC membrane, oriented with its polar carbonyl group towards the aqueous phase. This membrane position is only marginally altered in the presence of cholesterol. A previously reported result that ibuprofen is expelled from the membrane interface in cholesterol-containing DMPC bilayers could not be confirmed.
Keywords: NMR; cholesterol; fluorescence; ibuprofen; membrane interaction; membrane properties.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Cholesterol expels ibuprofen from the hydrophobic membrane core and stabilizes lamellar phases in lipid membranes containing ibuprofen.Soft Matter. 2015 Jun 28;11(24):4756-67. doi: 10.1039/c5sm00597c. Epub 2015 Apr 27. Soft Matter. 2015. PMID: 25915907
-
The influence of cholesterol on interactions and dynamics of ibuprofen in a lipid bilayer.Biochim Biophys Acta. 2014 Oct;1838(10):2431-8. doi: 10.1016/j.bbamem.2014.05.029. Epub 2014 Jun 6. Biochim Biophys Acta. 2014. PMID: 24911406
-
Octyl-beta-D-glucopyranoside partitioning into lipid bilayers: thermodynamics of binding and structural changes of the bilayer.Biophys J. 1997 Apr;72(4):1719-31. doi: 10.1016/S0006-3495(97)78818-0. Biophys J. 1997. PMID: 9083676 Free PMC article.
-
Vesicle-Based Assays to Study Membrane Interactions of Amyloid Peptides.Methods Mol Biol. 2019;1873:39-51. doi: 10.1007/978-1-4939-8820-4_3. Methods Mol Biol. 2019. PMID: 30341602 Review.
-
Cholesterol Effects on the Physical Properties of Lipid Membranes Viewed by Solid-state NMR Spectroscopy.Adv Exp Med Biol. 2019;1115:99-133. doi: 10.1007/978-3-030-04278-3_5. Adv Exp Med Biol. 2019. PMID: 30649757 Review.
Cited by
-
Altered Membrane Mechanics Provides a Receptor-Independent Pathway for Serotonin Action.Chemistry. 2021 May 12;27(27):7533-7541. doi: 10.1002/chem.202100328. Epub 2021 Mar 12. Chemistry. 2021. PMID: 33502812 Free PMC article.
-
Ibuprofen in a Lipid Bilayer: Nanoscale Spatial Arrangement.Membranes (Basel). 2022 Oct 30;12(11):1077. doi: 10.3390/membranes12111077. Membranes (Basel). 2022. PMID: 36363632 Free PMC article.
-
Comprehensive Insights into the Cholesterol-Mediated Modulation of Membrane Function through Molecular Dynamics Simulations.ArXiv [Preprint]. 2025 Apr 7:arXiv:2504.05564v1. ArXiv. 2025. Update in: Membranes (Basel). 2025 Jun 08;15(6):173. doi: 10.3390/membranes15060173. PMID: 40297234 Free PMC article. Updated. Preprint.
-
Mechanistic Understanding from Molecular Dynamics in Pharmaceutical Research 2: Lipid Membrane in Drug Design.Pharmaceuticals (Basel). 2021 Oct 19;14(10):1062. doi: 10.3390/ph14101062. Pharmaceuticals (Basel). 2021. PMID: 34681286 Free PMC article. Review.
-
Enhanced Stability and In Vitro Biocompatibility of Chitosan-Coated Lipid Vesicles for Indomethacin Delivery.Pharmaceutics. 2024 Dec 9;16(12):1574. doi: 10.3390/pharmaceutics16121574. Pharmaceutics. 2024. PMID: 39771553 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

