The inhibitory effect of some natural bioactive compounds against SARS-CoV-2 main protease: insights from molecular docking analysis and molecular dynamic simulation
- PMID: 32998618
- PMCID: PMC7544954
- DOI: 10.1080/10934529.2020.1826192
The inhibitory effect of some natural bioactive compounds against SARS-CoV-2 main protease: insights from molecular docking analysis and molecular dynamic simulation
Abstract
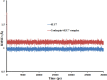
This work aimed at evaluating the inhibitory effect of ten natural bioactive compounds (1-10) as potential inhibitors of SARS-CoV-2-3CL main protease (PDB ID: 6LU7) and SARS-CoV main proteases (PDB IDs: 2GTB and 3TNT) by molecular docking analysis. The inhibitory effect of all studied compounds was studied with compared to some proposed antiviral drugs which currently used in COVID-19 treatment such as chloroquine, hydroxychloroquine, azithromycin, remdesivir, baloxvir, lopinavir, and favipiravir. Homology modeling and sequence alignment was computed to evaluate the similarity between the SARS-CoV-2-3CL main protease and other SARS-CoV receptors. ADMET properties of all studied compounds were computed and reported. Also, molecular dynamic (MD) simulation was performed on the compound which has the highest binding affinity inside 6LU7 obtained from molecular docking analysis to study it is stability inside receptor in explicit water solvent. Based on molecular docking analysis, we found that caulerpin has the highest binding affinity inside all studied receptors compared to other bioactive compounds and studied drugs. Our homology modeling and sequence alignment showed that SARS-CoV main protease (PDB ID: 3TNT) shares high similarity with 3CLpro (96.00%). Also, ADMET properties confirmed that caulerpin obeys Lipinski's rule and passes ADMET property, which make it a promising compound to act as a new safe natural drug against SARS-CoV-2-3CL main protease. Finally, MD simulation confirmed that the complex formed between caulerpin and 3CLpro is stable in water explicit and had no major effect on the flexibility of the protein throughout the simulations and provided a suitable basis for our study. Also, binding free energy between caulerpin and 6LU7 confirmed the efficacy of the caulerpin molecule against SARS-CoV-2 main protease. So, this study suggested that caulerpin could be used as a potential candidate in COVID-19 treatment.
Keywords: COVID-19 virus protease; MD simulation; caulerpin; molecular docking; natural products.
Figures




Similar articles
-
In silico identification of potential inhibitors of key SARS-CoV-2 3CL hydrolase (Mpro) via molecular docking, MMGBSA predictive binding energy calculations, and molecular dynamics simulation.PLoS One. 2020 Jul 24;15(7):e0235030. doi: 10.1371/journal.pone.0235030. eCollection 2020. PLoS One. 2020. PMID: 32706783 Free PMC article.
-
Optimization Rules for SARS-CoV-2 Mpro Antivirals: Ensemble Docking and Exploration of the Coronavirus Protease Active Site.Viruses. 2020 Aug 26;12(9):942. doi: 10.3390/v12090942. Viruses. 2020. PMID: 32859008 Free PMC article.
-
Interactions Between Remdesivir, Ribavirin, Favipiravir, Galidesivir, Hydroxychloroquine and Chloroquine with Fragment Molecular of the COVID-19 Main Protease with Inhibitor N3 Complex (PDB ID:6LU7) Using Molecular Docking.J Nanosci Nanotechnol. 2020 Dec 1;20(12):7311-7323. doi: 10.1166/jnn.2020.18955. J Nanosci Nanotechnol. 2020. PMID: 32711596
-
Design and Evaluation of Anti-SARS-Coronavirus Agents Based on Molecular Interactions with the Viral Protease.Molecules. 2020 Aug 27;25(17):3920. doi: 10.3390/molecules25173920. Molecules. 2020. PMID: 32867349 Free PMC article. Review.
-
Targeting the Dimerization of the Main Protease of Coronaviruses: A Potential Broad-Spectrum Therapeutic Strategy.ACS Comb Sci. 2020 Jun 8;22(6):297-305. doi: 10.1021/acscombsci.0c00058. Epub 2020 May 27. ACS Comb Sci. 2020. PMID: 32402186 Review.
Cited by
-
Viral inhibitors derived from macroalgae, microalgae, and cyanobacteria: A review of antiviral potential throughout pathogenesis.Algal Res. 2021 Jul;57:102331. doi: 10.1016/j.algal.2021.102331. Epub 2021 May 18. Algal Res. 2021. PMID: 34026476 Free PMC article.
-
Focus on Marine Animal Safety and Marine Bioresources in Response to the SARS-CoV-2 Crisis.Int J Mol Sci. 2022 Dec 1;23(23):15136. doi: 10.3390/ijms232315136. Int J Mol Sci. 2022. PMID: 36499463 Free PMC article. Review.
-
Exploring the inhibitory potential of novel bioactive compounds from mangrove actinomycetes against nsp10 the major activator of SARS-CoV-2 replication.Chem Zvesti. 2022;76(5):3051-3064. doi: 10.1007/s11696-021-01997-x. Epub 2022 Jan 27. Chem Zvesti. 2022. PMID: 35103034 Free PMC article.
-
Exploring the inhibitory potential of novel piperidine-derivatives against main protease (Mpro) of SARS-CoV-2: A hybrid approach consisting of molecular docking, MD simulations and MMPBSA analysis.J Mol Liq. 2023 Jul 15;382:121904. doi: 10.1016/j.molliq.2023.121904. Epub 2023 Apr 26. J Mol Liq. 2023. PMID: 37151376 Free PMC article.
-
Unveiling Berberine analogues as potential inhibitors of Escherichia coli FtsZ through machine learning molecular docking and molecular dynamics approach.Sci Rep. 2025 Apr 26;15(1):14668. doi: 10.1038/s41598-025-98835-x. Sci Rep. 2025. PMID: 40287515 Free PMC article.
References
-
- Li, Y.; Zhang, J.; Wang, N.; Li, H.; Shi, Y.; Guo, G.; Zou, Q.. Therapeutic Drugs Targeting 2019-nCoV Main Protease by High-Throughput Screening. bioRxiv 2020.
-
- Xu, X.; Chen, P.; Wang, J.; Feng, J.; Zhou, H.; Li, X.; Zhong, W.; Hao, P.. Evolution of the Novel Coronavirus from the Ongoing Wuhan Outbreak and Modeling of Its Spike Protein for Risk of Human Transmission. Sci. China Life Sci. 2020, 63, 457–460. DOI: 10.1007/s11427-020-1637-5. - DOI - PMC - PubMed
-
- Lu, I.-L.; Mahindroo, N.; Liang, P.-H.; Peng, Y.-H.; Kuo, C.-J.; Tsai, K.-C.; Hsieh, H.-P.; Chao, Y.-S.; Wu, S.-Y.. Structure-Based Drug Design and Structural Biology Study of Novel Nonpeptide Inhibitors of Severe Acute Respiratory Syndrome Coronavirus Main Protease. J. Med. Chem. 2006, 49, 5154–5161. DOI: 10.1021/jm060207o. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
