Effects of semaglutide on risk of cardiovascular events across a continuum of cardiovascular risk: combined post hoc analysis of the SUSTAIN and PIONEER trials
- PMID: 32998732
- PMCID: PMC7526237
- DOI: 10.1186/s12933-020-01106-4
Effects of semaglutide on risk of cardiovascular events across a continuum of cardiovascular risk: combined post hoc analysis of the SUSTAIN and PIONEER trials
Abstract
Background: Semaglutide is a glucagon-like peptide-1 (GLP-1) analog treatment for type 2 diabetes (T2D) available in subcutaneous (s.c.) and oral formulations. Two cardiovascular (CV) outcomes trials showed that in subjects with T2D at high risk of CV events there were fewer major adverse CV events (MACE; defined as CV death, non-fatal stroke, non-fatal myocardial infarction) with semaglutide than with placebo (hazard ratio [95% CI]: 0.74 [0.58;0.95] for once-weekly s.c. semaglutide and 0.79 [0.57;1.11] for once-daily oral semaglutide). However, there is little evidence for an effect of semaglutide on MACE in subjects not at high risk of CV events. This post hoc analysis examined CV effects of semaglutide in subjects across a continuum of baseline CV risk.
Methods: Data from the s.c. (SUSTAIN) and oral (PIONEER) semaglutide phase 3a clinical trial programs were combined according to randomized treatment (semaglutide or comparators) and analyzed to assess time to first MACE and its individual components. A CV risk model was developed with independent data from the LEADER trial (liraglutide vs placebo), considering baseline variables common to all datasets. Semaglutide data were analyzed to assess effects of treatment as a function of CV risk predicted using the CV risk prediction model.
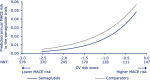
Results: The CV risk prediction model performed satisfactorily when applied to the semaglutide data set (area under the curve: 0.77). There was a reduced relative and absolute risk of MACE for semaglutide vs comparators across the entire continuum of CV risk. While the relative risk reduction tended to be largest with low CV risk score, the largest absolute risk reduction was for intermediate to high CV risk score. Similar results were seen for relative risk reduction of the individual MACE components and also when only placebo comparator data were included.
Conclusion: Semaglutide reduced the risk of MACE vs comparators across the continuum of baseline CV risk in a broad T2D population. Trial registrations ClinicalTrials.gov identifiers: NCT02054897, NCT01930188, NCT01885208, NCT02128932, NCT02305381, NCT01720446, NCT02207374, NCT02254291, NCT02906930, NCT02863328, NCT02607865, NCT02863419, NCT02827708, NCT02692716, NCT02849080, NCT03021187, NCT03018028, NCT03015220.
Keywords: Cardiovascular; MACE; Oral semaglutide; PIONEER; Risk prediction model; SUSTAIN; Subcutaneous semaglutide; Type 2 diabetes.
Conflict of interest statement
MH received advisory board fees from AstraZeneca, Boehringer Ingelheim, Janssen, Merck, Novo Nordisk and Roche; research grants from AstraZeneca, Merck and Novo Nordisk; and speaker fees from Boehringer Ingelheim, Janssen and Novo Nordisk. SCB received honoraria fees, teaching and research grants from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk and Sanofi; funding for development of educational programs from Elsevier and Medscape; he also provided expert advice from All-Wales Medicines Strategy Group and National Institute for Health and Care Excellence (NICE) UK; and is a shareholder of Glycosmedia. AGH was a full-time employee of Novo Nordisk for the majority of manuscript development, and is a Novo Nordisk shareholder. TM was a full-time employee of Novo Nordisk for the majority of manuscript development, and is a Novo Nordisk shareholder. SM is an employee of Novo Nordisk and owns stock in the company. IL received grant support from Merck, Mylan, Novo Nordisk, Pfizer and Sanofi, personal fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Intarcia, Janssen, Mannkind, Novo Nordisk, Sanofi, TARGETPharma and Valeritas; and non-financial support from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Jannsen, Novo Nordisk, Pfizer and Sanofi.
Figures


References
-
- Novo Nordisk. Ozempic® (semaglutide) Prescribing Information, 2020. https://www.novo-pi.com/ozempic.pdf. Accessed June 2020.
-
- Novo Nordisk. Rybelsus® (oral semaglutide) Prescribing information, 2020. https://www.novo-pi.com/rybelsus.pdf. Accessed June 2020.
-
- Buckley ST, Bækdal TA, Vegge A, Maarbjerg SJ, Pyke C, Ahnfelt-Rønne J, Madsen KG, Schéele SG, Alanentalo T, Kirk RK, et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci Transl Med. 2018;10(467):eaar7047. doi: 10.1126/scitranslmed.aar7047. - DOI - PubMed
-
- Granhall C, Donsmark M, Blicher TM, Golor G, Sondergaard FL, Thomsen M, Baekdal TA. Safety and pharmacokinetics of single and multiple ascending doses of the novel oral human GLP-1 analogue, oral semaglutide, in healthy subjects and subjects with type 2 diabetes. Clin Pharmacokinet. 2019;58(6):781–791. doi: 10.1007/s40262-018-0728-4. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

