Adaptive optics two-photon endomicroscopy enables deep-brain imaging at synaptic resolution over large volumes
- PMID: 32998883
- PMCID: PMC7527232
- DOI: 10.1126/sciadv.abc6521
Adaptive optics two-photon endomicroscopy enables deep-brain imaging at synaptic resolution over large volumes
Abstract
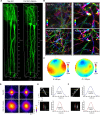
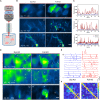
Optical deep-brain imaging in vivo at high resolution has remained a great challenge over the decades. Two-photon endomicroscopy provides a minimally invasive approach to image buried brain structures, once it is integrated with a gradient refractive index (GRIN) lens embedded in the brain. However, its imaging resolution and field of view are compromised by the intrinsic aberrations of the GRIN lens. Here, we develop a two-photon endomicroscopy by adding adaptive optics based on direct wavefront sensing, which enables recovery of diffraction-limited resolution in deep-brain imaging. A new precompensation strategy plays a critical role to correct aberrations over large volumes and achieve rapid random-access multiplane imaging. We investigate the neuronal plasticity in the hippocampus, a critical deep brain structure, and reveal the relationship between the somatic and dendritic activity of pyramidal neurons.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

