Early Change in Albuminuria with Canagliflozin Predicts Kidney and Cardiovascular Outcomes: A PostHoc Analysis from the CREDENCE Trial
- PMID: 32998938
- PMCID: PMC7790219
- DOI: 10.1681/ASN.2020050723
Early Change in Albuminuria with Canagliflozin Predicts Kidney and Cardiovascular Outcomes: A PostHoc Analysis from the CREDENCE Trial
Abstract
Background: The association between early changes in albuminuria and kidney and cardiovascular events is primarily based on trials of renin-angiotensin system blockade. It is unclear whether this association occurs with sodium-glucose cotransporter 2 inhibition.
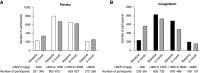
Methods: The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial enrolled 4401 patients with type 2 diabetes and CKD (urinary albumin-creatinine ratio [UACR] >300 mg/g). This post hoc analysis assessed canagliflozin's effect on albuminuria and how early change in albuminuria (baseline to week 26) is associated with the primary kidney outcome (ESKD, doubling of serum creatinine, or kidney death), major adverse cardiovascular events, and hospitalization for heart failure or cardiovascular death.
Results: Complete data for early change in albuminuria and other covariates were available for 3836 (87.2%) participants in the CREDENCE trial. Compared with placebo, canagliflozin lowered UACR by 31% (95% confidence interval [95% CI], 27% to 36%) at week 26, and significantly increased the likelihood of achieving a 30% reduction in UACR (odds ratio, 2.69; 95% CI, 2.35 to 3.07). Each 30% decrease in UACR over the first 26 weeks was independently associated with a lower hazard for the primary kidney outcome (hazard ratio [HR], 0.71; 95% CI, 0.67 to 0.76; P<0.001), major adverse cardiovascular events (HR, 0.92; 95% CI, 0.88 to 0.96; P<0.001), and hospitalization for heart failure or cardiovascular death (HR, 0.86; 95% CI, 0.81 to 0.90; P<0.001). Residual albuminuria levels at week 26 remained a strong independent risk factor for kidney and cardiovascular events, overall and in each treatment arm.
Conclusions: In people with type 2 diabetes and CKD, use of canagliflozin results in early, sustained reductions in albuminuria, which were independently associated with long-term kidney and cardiovascular outcomes.
Keywords: SGLT2 inhibitor; albuminuria; canagliflozin; kidney and cardiovascular outcomes.
Copyright © 2020 by the American Society of Nephrology.
Figures






References
-
- Eder S, Leierer J, Kerschbaum J, Rosivall L, Wiecek A, de Zeeuw D, et al. .: A prospective cohort study in patients with type 2 diabetes mellitus for validation of biomarkers (PROVALID) – study design and baseline characteristics. Kidney Blood Press Res 43: 181–190, 2018. - PubMed
-
- de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, et al. .: Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int 65: 2309–2320, 2004. - PubMed
-
- Heerspink HJ, Ninomiya T, Persson F, Brenner BM, Brunel P, Chaturvedi N, et al. .: Is a reduction in albuminuria associated with renal and cardiovascular protection? A post hoc analysis of the ALTITUDE trial. Diabetes Obes Metab 18: 169–177, 2016. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

