Sculpting Dendritic Spines during Initiation and Maintenance of Neuropathic Pain
- PMID: 32998955
- PMCID: PMC7531544
- DOI: 10.1523/JNEUROSCI.1664-20.2020
Sculpting Dendritic Spines during Initiation and Maintenance of Neuropathic Pain
Abstract
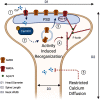
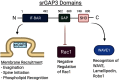
Accumulating evidence has established a firm role for synaptic plasticity in the pathogenesis of neuropathic pain. Recent advances have highlighted the importance of dendritic spine remodeling in driving synaptic plasticity within the CNS. Identifying the molecular players underlying neuropathic pain induced structural and functional maladaptation is therefore critical to understanding its pathophysiology. This process of dynamic reorganization happens in unique phases that have diverse pathologic underpinnings in the initiation and maintenance of neuropathic pain. Recent evidence suggests that pharmacological targeting of specific proteins during distinct phases of neuropathic pain development produces enhanced antinociception. These findings outline a potential new paradigm for targeted treatment and the development of novel therapies for neuropathic pain. We present a concise review of the role of dendritic spines in neuropathic pain and outline the potential for modulation of spine dynamics by targeting two proteins, srGAP3 and Rac1, critically involved in the regulation of the actin cytoskeleton.
Copyright © 2020 the authors.
Figures


Similar articles
-
Distinct roles of srGAP3-Rac1 in the initiation and maintenance phases of neuropathic pain induced by paclitaxel.J Physiol. 2020 Jun;598(12):2415-2430. doi: 10.1113/JP279525. Epub 2020 May 1. J Physiol. 2020. PMID: 32237255
-
Spinal Wnt5a Plays a Key Role in Spinal Dendritic Spine Remodeling in Neuropathic and Inflammatory Pain Models and in the Proalgesic Effects of Peripheral Wnt3a.J Neurosci. 2020 Aug 26;40(35):6664-6677. doi: 10.1523/JNEUROSCI.2942-19.2020. Epub 2020 Jul 2. J Neurosci. 2020. PMID: 32616667 Free PMC article.
-
Dendritic spine dysgenesis in neuropathic pain.Neurosci Lett. 2015 Aug 5;601:54-60. doi: 10.1016/j.neulet.2014.11.024. Epub 2014 Nov 20. Neurosci Lett. 2015. PMID: 25445354 Review.
-
Neuropathic pain memory is maintained by Rac1-regulated dendritic spine remodeling after spinal cord injury.J Neurosci. 2008 Dec 3;28(49):13173-83. doi: 10.1523/JNEUROSCI.3142-08.2008. J Neurosci. 2008. PMID: 19052208 Free PMC article.
-
Dendritic spine dysgenesis in neuropathic pain.Prog Mol Biol Transl Sci. 2015;131:385-408. doi: 10.1016/bs.pmbts.2014.12.001. Epub 2015 Feb 7. Prog Mol Biol Transl Sci. 2015. PMID: 25744680 Review.
Cited by
-
Characterisation of lamina I anterolateral system neurons that express Cre in a Phox2a-Cre mouse line.Sci Rep. 2021 Sep 9;11(1):17912. doi: 10.1038/s41598-021-97105-w. Sci Rep. 2021. PMID: 34504158 Free PMC article.
-
MicroRNA-138: an emerging regulator of skeletal development, homeostasis, and disease.Am J Physiol Cell Physiol. 2023 Dec 1;325(6):C1387-C1400. doi: 10.1152/ajpcell.00382.2023. Epub 2023 Oct 16. Am J Physiol Cell Physiol. 2023. PMID: 37842749 Free PMC article. Review.
-
Electroacupuncture inhibits dendritic spine remodeling through the srGAP3-Rac1 signaling pathway in rats with SNL.Biol Res. 2023 May 22;56(1):26. doi: 10.1186/s40659-023-00439-0. Biol Res. 2023. PMID: 37211600 Free PMC article.
-
Conditional Astrocyte Rac1KO Attenuates Hyperreflexia after Spinal Cord Injury.J Neurosci. 2024 Jan 3;44(1):e1670222023. doi: 10.1523/JNEUROSCI.1670-22.2023. J Neurosci. 2024. PMID: 37963762 Free PMC article.
-
A Possible Mechanism for Development of Working Memory Impairment in Male Mice Subjected to Inflammatory Pain.Neuroscience. 2022 Nov 1;503:17-27. doi: 10.1016/j.neuroscience.2022.09.007. Epub 2022 Sep 11. Neuroscience. 2022. PMID: 36100034 Free PMC article.
References
-
- Anthony H, Karel S (2009) Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci 10:647–658. - PubMed
-
- Bacon C, Endris V, Andermatt I, Niederkofler V, Waltereit R, Bartsch D, Stoeckli ET, Rappold G (2011) Evidence for a role of srGAP3 in the positioning of commissural axons within the ventrolateral funiculus of the mouse spinal cord. PLoS One 6:e19887. 10.1371/journal.pone.0019887 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
