Blockade of immune checkpoints in lymph nodes through locoregional delivery augments cancer immunotherapy
- PMID: 32998971
- PMCID: PMC8377700
- DOI: 10.1126/scitranslmed.aay3575
Blockade of immune checkpoints in lymph nodes through locoregional delivery augments cancer immunotherapy
Abstract
Systemic administration of immune checkpoint blockade (ICB) monoclonal antibodies (mAbs) can unleash antitumor functions of T cells but is associated with variable response rates and off-target toxicities. We hypothesized that antitumor efficacy of ICB is limited by the minimal accumulation of mAb within tissues where antitumor immunity is elicited and regulated, which include the tumor microenvironment (TME) and secondary lymphoid tissues. In contrast to systemic administration, intratumoral and intradermal routes of administration resulted in higher mAb accumulation within both the TME and its draining lymph nodes (LNs) or LNs alone, respectively. The use of either locoregional administration route resulted in pronounced T cell responses from the ICB therapy, which developed in the secondary lymphoid tissues and TME of treated mice. Targeted delivery of mAb to tumor-draining lymph nodes (TdLNs) alone was associated with enhanced antitumor immunity and improved therapeutic effects compared to conventional systemic ICB therapy, and these effects were sustained at reduced mAb doses and comparable to those achieved by intratumoral administration. These data suggest that locoregional routes of administration of ICB mAb can augment ICB therapy by improving immunomodulation within TdLNs.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures
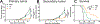

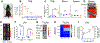
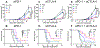

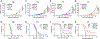
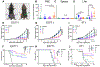

References
-
- Hargadon KM, Johnson CE, Williams CJ, Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol 62, 29–39 (2018). - PubMed
-
- Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, Peggs KS, Ravetch JV, Allison JP, Quezada SA, Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J. Exp. Med 210, 1695–1710 (2013). - PMC - PubMed
-
- Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, Korman AJ, Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol. Res 1, 32–42 (2013). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

