The Race between Host Antiviral Innate Immunity and the Immune Evasion Strategies of Herpes Simplex Virus 1
- PMID: 32998978
- PMCID: PMC7528619
- DOI: 10.1128/MMBR.00099-20
The Race between Host Antiviral Innate Immunity and the Immune Evasion Strategies of Herpes Simplex Virus 1
Erratum in
-
Correction for Zhu and Zheng, "The Race between Host Antiviral Innate Immunity and the Immune Evasion Strategies of Herpes Simplex Virus 1".Microbiol Mol Biol Rev. 2023 Dec 20;87(4):e0010323. doi: 10.1128/mmbr.00103-23. Epub 2023 Oct 16. Microbiol Mol Biol Rev. 2023. PMID: 37843293 Free PMC article. No abstract available.
Abstract
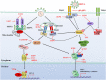
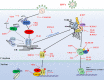
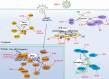
Herpes simplex virus 1 (HSV-1) is very successful in establishing acute and latent infections in humans by counteracting host antiviral innate immune responses. HSV-1 has evolved various strategies to evade host antiviral innate immunity and some cellular survival-associated pathways. Since there is still no vaccine available for HSV-1, a continuous update of information regarding the interaction between HSV-1 infection and the host antiviral innate immunity will provide novel insights to develop new therapeutic strategies for HSV-1 infection and its associated diseases. Here, we update recent studies about how HSV-1 evades the host antiviral innate immunity, specifically how HSV-1 proteins directly or indirectly target the adaptors in the antiviral innate immunity signaling pathways to downregulate the signal transduction. Additionally, some classical intracellular stress responses, which also play important roles in defense of viral invasion, will be discussed here. With a comprehensive review of evasion mechanisms of antiviral innate immunity by HSV-1, we will be able to develop potential new targets for therapies and a possible vaccine against HSV-1 infections.
Keywords: DDR; NLR; RIG-I/MDA5; TLR; antiviral immunity; apoptosis; cGAS-STING; herpes simplex virus; immune evasion.
Copyright © 2020 American Society for Microbiology.
Figures

References
-
- Saleh D, Sharma S. 2020. Herpes simplex type 1. StatPearls, Treasure Island, FL.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

