Non-Cell-Autonomous Activity of the Hemidesmosomal Protein BP180/Collagen XVII in Granulopoiesis in Humanized NC16A Mice
- PMID: 32998984
- PMCID: PMC7658030
- DOI: 10.4049/jimmunol.2000784
Non-Cell-Autonomous Activity of the Hemidesmosomal Protein BP180/Collagen XVII in Granulopoiesis in Humanized NC16A Mice
Abstract
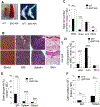
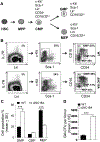
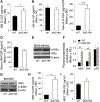
BP180 (also termed type XVII collagen) is a hemidesmosomal protein and plays a critical role in cell-cell matrix adhesion in the skin; however, its other biological functions are largely unclear. In this study, we generated a BP180 functional-deficient mouse strain by deleting its extracellular domain of humanized NC16A (termed ΔNC16A mice). We found that BP180 is expressed by bone marrow mesenchymal stem cells (BM-MSC), and its functional deficiency leads to myeloid hyperplasia. Altered granulopoiesis in ΔNC16A mice is through bone marrow stromal cells evidenced by bone marrow transplantation. Furthermore, the level of G-CSF in bone marrow and circulation were significantly increased in ΔNC16A mice as compared with wild-type mice. The increased G-CSF was accompanied by an increased activation of the NF-κB signaling pathway in bone marrow and BM-MSC of ΔNC16A mice. Blockade of G-CSF restored normal granulopoiesis in ΔNC16A mice. Inhibition of NF-κB signaling pathway significantly reduces the release of G-CSF from ΔNC16A BM-MSC in vitro and the level of serum G-CSF in ΔNC16A mice. To our knowledge, these findings provide the first direct evidence that BP180 plays an important role in granulopoiesis through regulating NF-κB signaling pathway in BM-MSC.
Copyright © 2020 by The American Association of Immunologists, Inc.
Figures



Similar articles
-
The dysfunction of BP180/collagen XVII in keratinocytes promotes melanoma progression.Oncogene. 2019 Dec;38(50):7491-7503. doi: 10.1038/s41388-019-0961-9. Epub 2019 Aug 21. Oncogene. 2019. PMID: 31435021 Free PMC article.
-
BP180 dysfunction triggers spontaneous skin inflammation in mice.Proc Natl Acad Sci U S A. 2018 Jun 19;115(25):6434-6439. doi: 10.1073/pnas.1721805115. Epub 2018 Jun 4. Proc Natl Acad Sci U S A. 2018. PMID: 29866844 Free PMC article.
-
Bullous pemphigoid induced by IgG targeting type XVII collagen non-NC16A/NC15A extracellular domains is driven by Fc gamma receptor- and complement-mediated effector mechanisms and is ameliorated by neonatal Fc receptor blockade.J Pathol. 2024 Feb;262(2):161-174. doi: 10.1002/path.6220. Epub 2023 Nov 6. J Pathol. 2024. PMID: 37929639
-
Collagen XVII/BP180: a collagenous transmembrane protein and component of the dermoepidermal anchoring complex.Clin Exp Dermatol. 2005 Nov;30(6):682-7. doi: 10.1111/j.1365-2230.2005.01937.x. Clin Exp Dermatol. 2005. PMID: 16197389 Review.
-
BP180 (type XVII collagen) and its role in cutaneous biology and disease.Adv Dermatol. 2003;19:37-71. Adv Dermatol. 2003. PMID: 14626817 Review.
Cited by
-
BP180/Collagen XVII: A Molecular View.Int J Mol Sci. 2021 Nov 12;22(22):12233. doi: 10.3390/ijms222212233. Int J Mol Sci. 2021. PMID: 34830116 Free PMC article. Review.
-
Rapamycin Promotes the Expansion of Myeloid Cells by Increasing G-CSF Expression in Mesenchymal Stem Cells.Front Cell Dev Biol. 2022 Mar 17;10:779159. doi: 10.3389/fcell.2022.779159. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35372343 Free PMC article.
References
-
- Koster J, Geerts D, Favre B, Borradori L, and Sonnenberg A. 2003. Analysis of the interactions between BP180, BP230, plectin and the integrin alpha6beta4 important for hemidesmosome assembly. J. Cell Sci 116: 387–399. - PubMed
-
- Margadant C, Frijns E, Wilhelmsen K, and Sonnenberg A. 2008. Regulation of hemidesmosome disassembly by growth factor receptors. Curr. Opin. Cell Biol 20: 589–596. - PubMed
-
- Diaz LA, Ratrie H, Saunders WS, Futamura S, Squiquera HL, Anhalt GJ, and Giudice GJ. 1990. Isolation of a human epidermal cDNA corresponding to the 180-kD autoantigen recognized by bullous pemphigoid and herpes gestationis sera: Immunolocalization of this protein to the hemidesmosome. J. Clin. Invest 86: 1088–1094. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

