Fluoxetine-induced recovery of serotonin and norepinephrine projections in a mouse model of post-stroke depression
- PMID: 32999279
- PMCID: PMC7527452
- DOI: 10.1038/s41398-020-01008-9
Fluoxetine-induced recovery of serotonin and norepinephrine projections in a mouse model of post-stroke depression
Abstract
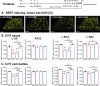
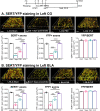
Chronic treatment with fluoxetine (FLX) is required for its antidepressant effects, but the role of serotonin (5-HT) axonal plasticity in FLX action is unknown. To address this, we examined mice with a stroke in the left medial prefrontal cortex (mPFC) resulting in persistent anxiety-like and depression-like behaviors and memory deficits as a model of post-stroke depression. Chronic treatment with FLX (but not exercise) completely reversed the behavioral phenotype and partially reversed changes in FosB-labeled cells in the mPFC, nucleus accumbens, septum, hippocampus, basolateral amygdala (BLA), and dorsal raphe. In these regions, 5-HT or norepinephrine (NE) innervation was quantified by staining for 5-HT or NE transporters, respectively. 5-HT synapses and synaptic triads were identified as synaptophysin-stained sites on 5-HT axons located proximal to gephyrin-stained or PSD95-stained spines. A week after stroke, 5-HT innervation was greatly reduced at the stroke site (left cingulate gyrus (CG) of the mPFC) and the left BLA. Chronically, 5-HT and NE innervation was reduced at the left CG, nucleus accumbens, and BLA, with no changes in other regions. In these areas, pre-synaptic and post-synaptic 5-HT synapses and triads to inhibitory (gephyrin+) sites were reduced, while 5-HT contacts at excitatory (PSD95+) sites were reduced in the CG and prelimbic mPFC. Chronic FLX, but not exercise, reversed these reductions in 5-HT innervation but incompletely restored NE projections. Changes in 5-HT innervation were verified using YFP staining in mice expressing YFP-tagged channelrhodopsin in 5-HT neurons. Thus, FLX-induced 5-HT axonal neuroplasticity of forebrain projections may help mediate recovery from brain injury.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Chronic Desipramine Reverses Deficits in Cell Activity, Norepinephrine Innervation, and Anxiety-Depression Phenotypes in Fluoxetine-Resistant cF1ko Mice.J Neurosci. 2024 Jan 17;44(3):e1147232023. doi: 10.1523/JNEUROSCI.1147-23.2023. J Neurosci. 2024. PMID: 38050173 Free PMC article.
-
Chronic Fluoxetine Induces Activity Changes in Recovery From Poststroke Anxiety, Depression, and Cognitive Impairment.Neurotherapeutics. 2018 Jan;15(1):200-215. doi: 10.1007/s13311-017-0590-3. Neurotherapeutics. 2018. PMID: 29204954 Free PMC article.
-
Maternal fluoxetine impairs synaptic transmission and plasticity in the medial prefrontal cortex and alters the structure and function of dorsal raphe nucleus neurons in offspring mice.Pharmacol Biochem Behav. 2024 Nov;244:173849. doi: 10.1016/j.pbb.2024.173849. Epub 2024 Aug 13. Pharmacol Biochem Behav. 2024. PMID: 39142357
-
Monoamine neurocircuitry in depression and strategies for new treatments.Prog Neuropsychopharmacol Biol Psychiatry. 2013 Aug 1;45:54-63. doi: 10.1016/j.pnpbp.2013.04.009. Epub 2013 Apr 19. Prog Neuropsychopharmacol Biol Psychiatry. 2013. PMID: 23602950 Review.
-
Rewiring of the Serotonin System in Major Depression.Front Psychiatry. 2021 Dec 16;12:802581. doi: 10.3389/fpsyt.2021.802581. eCollection 2021. Front Psychiatry. 2021. PMID: 34975594 Free PMC article. Review.
Cited by
-
Serotonergic Regulation in Alzheimer's Disease.Int J Mol Sci. 2025 May 29;26(11):5218. doi: 10.3390/ijms26115218. Int J Mol Sci. 2025. PMID: 40508026 Free PMC article. Review.
-
Chronic Desipramine Reverses Deficits in Cell Activity, Norepinephrine Innervation, and Anxiety-Depression Phenotypes in Fluoxetine-Resistant cF1ko Mice.J Neurosci. 2024 Jan 17;44(3):e1147232023. doi: 10.1523/JNEUROSCI.1147-23.2023. J Neurosci. 2024. PMID: 38050173 Free PMC article.
-
Microglia Involves in the Immune Inflammatory Response of Poststroke Depression: A Review of Evidence.Oxid Med Cell Longev. 2022 Aug 2;2022:2049371. doi: 10.1155/2022/2049371. eCollection 2022. Oxid Med Cell Longev. 2022. Retraction in: Oxid Med Cell Longev. 2024 Jan 9;2024:9860462. doi: 10.1155/2024/9860462. PMID: 35958023 Free PMC article. Retracted. Review.
-
Reduction of p11 in dorsal raphe nucleus serotonergic neurons mediates depression-like behaviors.Transl Psychiatry. 2023 Nov 22;13(1):359. doi: 10.1038/s41398-023-02664-3. Transl Psychiatry. 2023. PMID: 37993435 Free PMC article.
-
Neurotransmitters' white matter mapping unveils the neurochemical fingerprints of stroke.Nat Commun. 2025 Mar 15;16(1):2555. doi: 10.1038/s41467-025-57680-2. Nat Commun. 2025. PMID: 40089467 Free PMC article.
References
-
- WHO. Depression and Other Common Mental Disorders: Global Health Estimates (World Health Organization, Geneva, Switzerland, 2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

