NOTCH1 is critical for fibroblast-mediated induction of cardiomyocyte specialization into ventricular conduction system-like cells in vitro
- PMID: 32999360
- PMCID: PMC7527973
- DOI: 10.1038/s41598-020-73159-0
NOTCH1 is critical for fibroblast-mediated induction of cardiomyocyte specialization into ventricular conduction system-like cells in vitro
Abstract
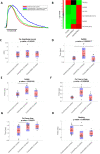
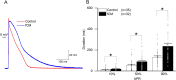
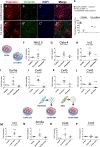
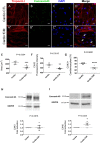
Cardiac fibroblasts are present throughout the myocardium and are enriched in the microenvironment surrounding the ventricular conduction system (VCS). Several forms of arrhythmias are linked to VCS abnormalities, but it is still unclear whether VCS malformations are cardiomyocyte autonomous or could be linked to crosstalk between different cell types. We reasoned that fibroblasts influence cardiomyocyte specialization in VCS cells. We developed 2D and 3D culture models of neonatal rat cardiac cells to assess the influence of cardiac fibroblasts on cardiomyocytes. Cardiomyocytes adjacent to cardiac fibroblasts showed a two-fold increase in expression of VCS markers (NAV1.5 and CONTACTIN 2) and calcium transient duration, displaying a Purkinje-like profile. Fibroblast-conditioned media (fCM) was sufficient to activate VCS-related genes (Irx3, Scn5a, Connexin 40) and to induce action potential prolongation, a hallmark of Purkinge phenotype. fCM-mediated response seemed to be spatially-dependent as cardiomyocyte organoids treated with fCM had increased expression of connexin 40 and NAV1.5 primarily on its outer surface. Finally, NOTCH1 activation in both cardiomyocytes and fibroblasts was required for connexin 40 up-regulation (a proxy of VCS phenotype). Altogether, we provide evidence that cardiac fibroblasts influence cardiomyocyte specialization into VCS-like cells via NOTCH1 signaling in vitro.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Characterizing the role of atrial natriuretic peptide signaling in the development of embryonic ventricular conduction system.Sci Rep. 2018 May 2;8(1):6939. doi: 10.1038/s41598-018-25292-0. Sci Rep. 2018. PMID: 29720615 Free PMC article.
-
Cardiac fibroblast paracrine factors alter impulse conduction and ion channel expression of neonatal rat cardiomyocytes.Cardiovasc Res. 2009 Sep 1;83(4):688-97. doi: 10.1093/cvr/cvp164. Epub 2009 May 28. Cardiovasc Res. 2009. PMID: 19477968 Free PMC article.
-
Age-dependent functional crosstalk between cardiac fibroblasts and cardiomyocytes in a 3D engineered cardiac tissue.Acta Biomater. 2017 Jun;55:120-130. doi: 10.1016/j.actbio.2017.04.027. Epub 2017 Apr 25. Acta Biomater. 2017. PMID: 28455218 Free PMC article.
-
Electrical consequences of cardiac myocyte: fibroblast coupling.Biochem Soc Trans. 2015 Jun;43(3):513-8. doi: 10.1042/BST20150035. Biochem Soc Trans. 2015. PMID: 26009200 Review.
-
Influence of mechanical stress on fibroblast-myocyte interactions in mammalian heart.J Mol Cell Cardiol. 2014 May;70:27-36. doi: 10.1016/j.yjmcc.2013.12.020. Epub 2014 Jan 2. J Mol Cell Cardiol. 2014. PMID: 24389344 Review.
Cited by
-
Multicellular 3D Models for the Study of Cardiac Fibrosis.Int J Mol Sci. 2022 Oct 1;23(19):11642. doi: 10.3390/ijms231911642. Int J Mol Sci. 2022. PMID: 36232943 Free PMC article. Review.
-
Pacemaker translocations and power laws in 2D stem cell-derived cardiomyocyte cultures.PLoS One. 2022 Mar 14;17(3):e0263976. doi: 10.1371/journal.pone.0263976. eCollection 2022. PLoS One. 2022. PMID: 35286321 Free PMC article.
-
The Roles of Cardiac Fibroblasts and Endothelial Cells in Myocarditis.Front Cardiovasc Med. 2022 Apr 7;9:882027. doi: 10.3389/fcvm.2022.882027. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35463742 Free PMC article. Review.
-
Bioengineering Systems for Modulating Notch Signaling in Cardiovascular Development, Disease, and Regeneration.J Cardiovasc Dev Dis. 2021 Sep 30;8(10):125. doi: 10.3390/jcdd8100125. J Cardiovasc Dev Dis. 2021. PMID: 34677194 Free PMC article. Review.
-
Adriamycin induces cardiac fibrosis in mice via PRMT5-mediated cardiac fibroblast activation.Acta Pharmacol Sin. 2023 Mar;44(3):573-583. doi: 10.1038/s41401-022-00963-x. Epub 2022 Sep 2. Acta Pharmacol Sin. 2023. PMID: 36056082 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

