Micromanipulation of prophase I chromosomes from mouse spermatocytes reveals high stiffness and gel-like chromatin organization
- PMID: 32999386
- PMCID: PMC7528058
- DOI: 10.1038/s42003-020-01265-w
Micromanipulation of prophase I chromosomes from mouse spermatocytes reveals high stiffness and gel-like chromatin organization
Abstract
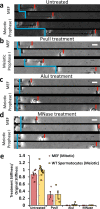
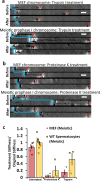
Meiosis produces four haploid cells after two successive divisions in sexually reproducing organisms. A critical event during meiosis is construction of the synaptonemal complex (SC), a large, protein-based bridge that physically links homologous chromosomes. The SC facilitates meiotic recombination, chromosome compaction, and the eventual separation of homologous chromosomes at metaphase I. We present experiments directly measuring physical properties of captured mammalian meiotic prophase I chromosomes. Mouse meiotic chromosomes are about ten-fold stiffer than somatic mitotic chromosomes, even for genetic mutants lacking SYCP1, the central element of the SC. Meiotic chromosomes dissolve when treated with nucleases, but only weaken when treated with proteases, suggesting that the SC is not rigidly connected, and that meiotic prophase I chromosomes are a gel meshwork of chromatin, similar to mitotic chromosomes. These results are consistent with a liquid- or liquid-crystal SC, but with SC-chromatin stiff enough to mechanically drive crossover interference.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

