Structural basis for pH gating of the two-pore domain K+ channel TASK2
- PMID: 32999458
- PMCID: PMC8628578
- DOI: 10.1038/s41586-020-2770-2
Structural basis for pH gating of the two-pore domain K+ channel TASK2
Abstract
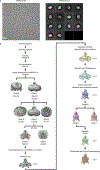
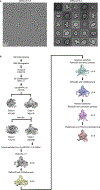
TASK2 (also known as KCNK5) channels generate pH-gated leak-type K+ currents to control cellular electrical excitability1-3. TASK2 is involved in the regulation of breathing by chemosensory neurons of the retrotrapezoid nucleus in the brainstem4-6 and pH homeostasis by kidney proximal tubule cells7,8. These roles depend on channel activation by intracellular and extracellular alkalization3,8,9, but the mechanistic basis for TASK2 gating by pH is unknown. Here we present cryo-electron microscopy structures of Mus musculus TASK2 in lipid nanodiscs in open and closed conformations. We identify two gates, distinct from previously observed K+ channel gates, controlled by stimuli on either side of the membrane. Intracellular gating involves lysine protonation on inner helices and the formation of a protein seal between the cytoplasm and the channel. Extracellular gating involves arginine protonation on the channel surface and correlated conformational changes that displace the K+-selectivity filter to render it nonconductive. These results explain how internal and external protons control intracellular and selectivity filter gates to modulate TASK2 activity.
Figures






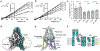
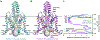


References
-
- Reyes R et al. Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. J Biol Chem 273, 30863–30869 (1998). - PubMed
-
- Enyedi P & Czirják G Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev 90, 559–605 (2010). - PubMed
-
- Niemeyer MI, Cid LP, Barros LF & Sepúlveda FV Modulation of the two-pore domain acid-sensitive K+ channel TASK-2 (KCNK5) by changes in cell volume. J Biol Chem 276, 43166–43174 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

