Electrochemical Detection of Acetaminophen with Silicon Nanowires
- PMID: 32999580
- PMCID: PMC7522788
Electrochemical Detection of Acetaminophen with Silicon Nanowires
Abstract
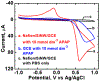
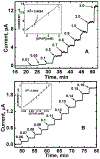
Acetaminophen (APAP) is an antipyretic, analgesic agent, the overdose of which during medical treatment poses a risk for liver failure. Hence, it is important to develop methods to monitor physiological APAP levels to avoid APAP. Here, we report an efficient, selective electrochemical APAP sensor made from depositing silicon nanowires (SiNWs) onto glassy carbon electrodes (GCEs). Electrocatalytic activity of the SiNW/GCE sensors was monitored under varying pH and concentrations of APAP using cyclic voltammetry (CV) and chronoamperometry (CA). CV of the SiNWs at 0.5 to 13 mmol dm-3 APAP concentrations was used to determine the oxidation and reduction potential of APAP. The selective detection of APAP was then demonstrated using CA at +0.568 V vs Ag/AgCl, where APAP is fully oxidized, in the 0.01 to 3 mmol dm-3 concentration range with potentially-interfering species. The SiNW sensor has the ability to detect APAP well within the detection limits for APAP toxicity, showing promise as a practical biosensor.
Keywords: acetaminophen; biosensor; silicon nanowires; toxicity monitoring.
Figures




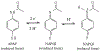
Similar articles
-
A robust electrochemical sensing based on bimetallic metal-organic framework mediated Mo2C for simultaneous determination of acetaminophen and isoniazid.Anal Chim Acta. 2020 Nov 1;1136:99-105. doi: 10.1016/j.aca.2020.08.044. Epub 2020 Aug 27. Anal Chim Acta. 2020. PMID: 33081955
-
Development of an electrochemical sensor for the quantification of ascorbic acid and acetaminophen in pharmaceutical samples.J Pharm Biomed Anal. 2024 Oct 15;249:116334. doi: 10.1016/j.jpba.2024.116334. Epub 2024 Jul 3. J Pharm Biomed Anal. 2024. PMID: 38976964
-
Simultaneous determination of N-acetyl-p-aminophenol and p-aminophenol with poly(3,4-ethylenedioxythiophene) modified glassy carbon electrode.Talanta. 2011 Sep 15;85(3):1376-82. doi: 10.1016/j.talanta.2011.06.019. Epub 2011 Jun 17. Talanta. 2011. PMID: 21807198
-
Silicon nanowires as field-effect transducers for biosensor development: a review.Anal Chim Acta. 2014 May 12;825:1-25. doi: 10.1016/j.aca.2014.03.016. Epub 2014 Mar 15. Anal Chim Acta. 2014. PMID: 24767146 Review.
-
Electrochemical methods for the determination of acetaminophen in biological matrices: A critical review in the clinical field.Anal Chim Acta. 2025 Jan 2;1333:343243. doi: 10.1016/j.aca.2024.343243. Epub 2024 Sep 13. Anal Chim Acta. 2025. PMID: 39615920 Review.
Cited by
-
Construction of a novel electrochemical sensor based on biomass material nanocellulose and its detection of acetaminophen.RSC Adv. 2022 Sep 28;12(43):27736-27745. doi: 10.1039/d2ra04125a. eCollection 2022 Sep 28. RSC Adv. 2022. PMID: 36320243 Free PMC article.
References
-
- Chen TS, Huang KL. Int. J. Electrochem. Sci 2012, 7, 6877–6892.
-
- Choi K, Kim Y, Park J, Park CK, Kim MY, Kim P . Sci. Total Environ 2008,405, 120–128. - PubMed
-
- Andreozzi R, Raffaele M, Nicklas P. Chemosphere 2003, 50, 1319–1330. - PubMed
-
- Nakata H, Kannan K, Jones PD, Giesy JP. Chemosphere 2005, 58, 759–766. - PubMed
-
- Brown KD, Kulis J, Thomson B, Chapman TH, Mawhinney DB. Sci. Total Environ 2006, 366, 772–783. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
