Obesity, estrogens and adipose tissue dysfunction - implications for pulmonary arterial hypertension
- PMID: 32999709
- PMCID: PMC7506791
- DOI: 10.1177/2045894020952023
Obesity, estrogens and adipose tissue dysfunction - implications for pulmonary arterial hypertension
Abstract
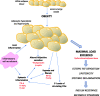
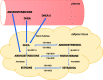
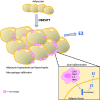
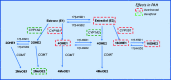
Obesity is a prevalent global public health issue characterized by excess body fat. Adipose tissue is now recognized as an important endocrine organ releasing an abundance of bioactive adipokines including, but not limited to, leptin, adiponectin and resistin. Obesity is a common comorbidity amongst pulmonary arterial hypertension patients, with 30% to 40% reported as obese, independent of other comorbidities associated with pulmonary arterial hypertension (e.g. obstructive sleep apnoea). An 'obesity paradox' has been observed, where obesity has been associated with subclinical right ventricular dysfunction but paradoxically may confer a protective effect on right ventricular function once pulmonary hypertension develops. Obesity and pulmonary arterial hypertension share multiple pathophysiological mechanisms including inflammation, oxidative stress, elevated leptin (proinflammatory) and reduced adiponectin (anti-inflammatory). The female prevalence of pulmonary arterial hypertension has instigated the hypothesis that estrogens may play a causative role in its development. Adipose tissue, a major site for storage and metabolism of sex steroids, is the primary source of estrogens and circulating estrogens levels which are elevated in postmenopausal women and men with pulmonary arterial hypertension. This review discusses the functions of adipose tissue in both health and obesity and the links between obesity and pulmonary arterial hypertension. Shared pathophysiological mechanisms and the contribution of specific fat depots, metabolic and sex-dependent differences are discussed.
Keywords: aromatase; metabolic syndrome; sex differences.
© The Author(s) 2020.
Figures
References
-
- Poms AD, Turner M, Farber HW, et al. Comorbid conditions and outcomes in patients with pulmonary arterial hypertension: a REVEAL registry analysis. Chest 2013; 144: 169–176. - PubMed
-
- Weatherald J, Huertas A, Boucly A, et al. Association between BMI and obesity with survival in pulmonary arterial hypertension. Chest 2018; 154: 872–881. - PubMed
-
- Haque AK, Gadre S, Taylor J, et al. Pulmonary and cardiovascular complications of obesity: an autopsy study of 76 obese subjects. Arch Pathol Lab Med 2008; 132: 1397–1404. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

